시판 농자재의 식균성 천적 노랑무당벌레(Illeis koebelei)에 대한 독성
초록
본 연구는 흰가루병원균 포식성 곤충인 노랑무당벌레 (Illeis koebelei)에 대한 시판 농자재의 독성을 분석한 최초의 연구로써 흰가루병 종합적관리의 기초자료를 확보하고자 수행하였다. 오이에 등록된 살충제와 유기농업자재를 대상으로 노랑무당벌레의 유충과 성충에 대한 독성을 검정한 결과, bifenthrin + imidacloprid (WP), acetamiprid + indoxacarb (WP), acetamiprid + etopheprox (WP)약제들은 IOBC 기준을 적용할 경우 Class 4 (harmful)에 속하는 높은 독성을 나타내었다. 단기간 독성평가시 저독성 살충제였던 spiromesifen (SC)은 노랑무당벌레 3령 유충과 갓 우화한 성충이 흰가루병원균과 동시에 섭식하더라도 생존율과 번식력에는 영향이 적었던 반면, pyriproxyfen (EC)의 경우는 유충의 용화율과 성충 번식력을 크게 떨어뜨리는 것으로 나타났다. 한편 유기농업자재인 큐팩트 (a.i. Ampelomyces quisqualis 94013)와 탑시드 (a.i. Paenibacillus polymyxa AC-1)는 3령 유충과 성충에 독성을 보이지 않았으며, 비티원 (Bacillus thuringiensis)은 갓 우화한 성충의 생존율과 번식력에 영향을 미치지 않았다. 따라서, 천적에 대한 독성 평가시 노출 이후 생존율과 번식력에 대한 장기적 검토가 필요하며, 위의 저독성 농자재들은 오이 흰가루병 종합적 방제에 노랑무당벌레와 함께 이용이 가능할 것으로 사료된다.
Abstract
This study is the first to report the toxicity of pesticides to mycophagous ladybird, Illeis koebelei, which feeds on powdery mildew fungi. We investigated the selective toxicities of synthetic or environment-friendly biopesticides to I. koebelei for integrated powdery mildew management programs. Three synthetic insecticides, bifenthrin + imidacloprid WP, acetamiprid + indoxacarb WP, and acetamiprid + etofenprox WP were very toxic (IOBC classification, Class 4) to I. koebelei. Spiromesifen SC showed low toxicity to the survival and fecundity of I. koebelei when the third instar larvae or newly emerged adults were exposed to this pesticide via feeding with spiromesifen SC-treated cucumber powdery mildew. Pyriproxyfen EC showed very high residual toxicity, and the pupation rate and fecundity decreased significantly. Many environment-friendly biopesticides restricted the population of I. koebelei. However, Q pact (a.i. Ampelomyces quisqualis 94013) and Top seed (a.i. Paenibacillus polymyxa AC-1) showed toxicity to I. koebelei larvae. BT one (a.i. Bacillus thuringiensis) showed no residual toxicity on the fecundity of I. koebelei adults.
Keywords:
Ladybeetle, Illeis koebelei, Mycophagous, Pesticide, Toxicity키워드:
무당벌레, Illeis koebelei, 식균성, 농자재, 독성Introduction
Pesticide use results in the unavoidable exposure of natural enemies of pests to these pesticides. Therefore, using of selective pesticides that have low toxicity to natural enemies is essential for the conservation of natural enemy populations (Tanaka et al., 2000). Thus, the use of selective pesticides is important in integrated pest management (IPM). The International Organization for Biological Control (IOBC) is active in identifying pesticides compatible with biological control. Based on IOBC classification, the effect of pesticides on natural enemy is categorized as Class 1 (harmless), Class 2 (slightly harmful), Class 3 (moderately harmful) and Class 4 (harmful) toxicity levels.
Powdery mildew disease is the most common and economically important plant disease in agricultural ecosystems worldwide (Amano, 1986). This disease damages a wide range of agricultural plant species (Glawe, 2008). The management of powdery mildew heavily relies on fungicides. However, owing to various adverse effects, such as development of pesticide resistance, environmental pollution, and killing of natural enemies (Razdan and Sabitha, 2009), the application of biological agents including microorganisms, and mycophagous arthropods has been increasingly studied (Bhattacharjee et al., 1994; English-Loeb et al., 2007; Lee et al., 2007; Romero et al., 2007; Segarra, 2009; Hegazi and El-Kot, 2010).
Of the Coccinellid group, mycophagous ladybeetles in the tribe Halyziini are considered biological control agents against powdery mildew. Halyziini ladybeetles feed primarily on powdery mildews and are distributed worldwide in regions where powdery mildews commonly occur (Sasaji, 1998; Giorgi et al., 2009; Sutherland and Parrella, 2009; Tabata et al., 2011). Mycophagous ladybird, Illeis koebelei is generally distributed in Asian countries, including China, Japan, Korea, Philippines, and Taiwan (Kim et al., 1994; Recuenco-Adorada and Gapud, 1998; Takeuchi et al., 2000; Lin et al., 2006; Wu et al., 2011). A recent study by Lee et al (2015) reported that I. koebelei was found from early July to early November in Korea, and has the potential for controlling cucumber powdery mildew.
Despite the broad distribution of mycophagous I. koebelei and its potential as a biological agent against powdery mildew disease in Korea, no information is available on the toxicity of pesticides to I. koebelei. Information on pesticide toxicity to I. koebelei would help farmers to select the appropriate pesticides for the chemical control of other pest and to develop a successful IPM program to control cucumber powdery mildew using this mycophagous ladybeetle. Therefore, in this study, we aimed to determine the toxicity of pesticides, which are, commonly used in cucumber production in Korea, to I. koebelei. The results of this study would provide a guideline for selecting appropriate pesticides for controlling powdery mildew using I. koebelei.
Materials and Methods
Rearing of I. koebelei
I. koebelei and cucumber powdery mildews were collected from pear orchards and cucumber plants in commercial greenhouses, respectively in Gyeonggi-do, Korea. I. koebelei was cultured on cucumber seedlings infected with powdery mildew in laboratory at 24 ± 1oC, 60-80% (RH) and a photoperiod of 16:8 (L:D) h. Approximately 30 adult I. koebelei were placed in acrylic containers (30 × 30 × 30 cm) with two pots of cucumber seedlings infected with powdery mildew. The eggs laid on cucumber leaves were transferred to a translucent plastic cage (232 × 165 × 95 mm) with ventilation holes on the sides. A wet paper-towel was placed at the bottom of cage to provide moisture. Neonates were moved to cucumber leaf infected with powdery mildew by using a soft brush. The larvae were always served with new cucumber seedlings before the exhaustion of food. The pupae were placed in acrylic containers until eclosion.
Pesticides tested
Sixteen synthetic insecticides (Table 1) for controlling Trialeurodes vaporariorum or Bemisia tabaci, and 22 environment-friendly commercial pesticides (Table 2), which are generally used in cucumber cultivation in Korea, were tested for toxicity to I. koebelei. All pesticides were diluted in water to the respective field-recommended application rate. The concentration of each pesticide was selected based on the 2012 Agrochemicals Use Guide Book (KCPA, 2012).
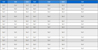
List of commercial synthetic insecticides tested for toxicity against mycophagous ladybird Illeis koebelei
Toxicity bioassay
To test the toxicity of the 38 pesticides listed above, the third instar larvae and adults of I. koebelei (<48 h old) were exposed to pesticides at the recommended field application rate of each commercial formulation. The larvae were sprayed with pesticide or water control until the body was soaked, and then placed in the insect breeding dish (Ø 150 × H 73 mm, ventilation allowed). The adults were dipped in pesticide or water control for 5 s and placed in the insect breeding dish (Ø 150 × H 73 mm, ventilation allowed). Cucumber leaf disc (Ø 100 mm) infected with powdery mildew was provided in the dish as a food source. The experiment was repeated three times with 15 individuals in each replicate for each treatment. Kimwipe (Kimberly- Clarke®, Kimwipes® EX-L) was placed at the bottom of the dish and moistened with water every day. Mortality was assessed 48 h after treatment. An insect was recorded as dead if it did not move or could not turn the body by itself when touched.
Pesticides that showed low toxicity to I. koebelei were further tested to evaluate the residual toxicity on survival and fecundity of I. koebelei at the same concentration. For the evaluation of residual toxicity to larvae, 30 third instar larvae were provided with cucumber leaves infected with powdery mildew, which had been exposed to pesticides. The amount of leaves provided was sufficient to feed the larva to pupation. After adult emergence, 10 adult couples were provided with pesticide-free cucumber seedlings, and the number of eggs laid by female adults was investigated for 20 days after the pre-oviposition period. For the evaluation of residual toxicity to adults, 15 adult couples (<24 h) were treated, and the survival rate, pre-oviposition period and fecundity were investigated by the same method.
All experiments were conducted in the laboratory at 24 ± 1oC, 60-80% RH and a photoperiod of 16:8 (L:D) h.
Statistical analysis
The mortalities (%) caused by synthetic insecticides and commercial environment-friendly pesticides were corrected for control mortality using the Abbott’s correction formula. Then, the mortalities (%) were transformed using the arcsine square root function prior to analysis of variance (ANOVA) test. The survival rate (%), pre-oviposition period, and fecundity of I. koebelei adults by the residual toxicity of pesticides, and pupation rate (%), emergence rate (%), preoviposition period, and fecundity of I. koebelei by the residual toxicity of pesticides were analyzed by one-way ANOVA. The means were separated with the Tukey’s Studentized Range (HSD) Test (SAS Institute, 2008).
Results
The effects of 16 synthetic insecticides on the mortality of I. koebelei under laboratory conditions are shown in Table 3. The susceptibility of I. koebelei to insecticides varied according to the insecticide and developmental stages. In general, the larvae were more susceptible to pesticides than the adults were. Neonicotinoid insecticides and a mixture chemicals, such as acetamiprid WP, acetamiprid + indoxacarb WP, acetamiprid + etofenprox WP, bifenthrin + imidacloprid WP, clothianidin SC, and dinotefuran WP showed strong toxicity to I. koebelei. However, thiamethoxam WG was relatively less toxic to I. koebelei than other neonicotinoid insecticides. In particular, thiametoxam WG showed very less toxic effect on adult I. koebelei. Pyrethroid insecticides and a mixture with pyrethroids, such as bifenthrin + imidacloprid WP, deltamethrin EC and gamma-cyhalothrin CS were also highly toxic to I. koebelei, regardless of its developmental stages. The insect growth regulator, pyriproxyfen EC and the new class of spirocyclic tetronic acids, spiromesifen SC, were less toxic to I. koebelei with < 20% mortality. Other insect growth regulators, bistrifluron EC and novaluron SC, were also less toxic to adult I. koebelei, but were highly toxic to its larvae
The effects of 22 environment-friendly pesticides on the mortality of I. koebelei under laboratory conditions are presented in Table 4. Most of the biological or botanical pesticides were more toxic to larvae than adults of I. koebelei similar to synthetic pesticides. Several botanical or biopesticides (e.g., Barogaru alpha, Bijin alpha, Eungaetan alpha, Eungsami, Nobug, Suncho) were highly toxic to larvae, or adult, or both stages of I. koebelei. However, Barogaru, Bijin alpha and Neem seed oil showed low toxic effects on adult I. koebelei. Among the biological or botanical pesticides tested, six commercial products (Barojin alpha, BT one, Daeyou ecocide, Q pact, Solbitchae, and Top seed) showed low mortality against the larvae and adults of I. koebelei, with < 20% mortality.

Effects of commercial synthetic insecticides on mortality of Illeis koebelei under laboratory conditions

Effects of commercial environment-friendly pesticides on mortality of Illeis koebelei under laboratory conditions
The effects of residual toxicity of several pesticides, which are less toxic to I. koebelei, on I. koebelei adults are presented in Table 5. The synthetic pesticides pyriproxyfen EC and spiromesifen SC showed low residual toxicity to I. koebele, in terms of mortality and pre-oviposition period. However, pyriproxyfen EC decreased fecundity significantly. The biopesticides BT one and Solbitchae did not show significant residual toxicity.

Effects of residual toxicity of several pesticides on the survival and fecundity adult Illeis koebelei
The effects of residual toxicity of several pesticides, which are less toxic to I. koebelei, on I. koebelei larvae are presented in Table 6. The synthetic pesticide pyriproxyfen EC showed significant residual toxicity, resulting in unsuccessful pupation. Another synthetic pesticide spiromesifen SC showed a rather low residual toxicity, resulting in 70% pupation rate. In addition two environment-friendly pesticides, BT one and Solbitchae, did not affect survival and showed low residual toxicity on fecundity of I. koebelei. Meanwhile, Solbitchae somewhat shortened the pre-oviposition period and decreased the fecundity of adults.
Discussion
This study is the first to report the toxicity of pesticides to the mycophagous predator, I. koebelei, of powdery mildew of agricultural crops. The pesticides tested are synthetic or environment-friendly products, and are used for controlling insect or microbial pests on cucumber in Korea. Many studies on toxicity against the multicolored Asian ladybeetle, Harmonia axyridis Pallas have been conducted with various insecticides, biopesticides, fungicides, herbicides, and insectresistant transgenic crops (Koch, 2003). Cho et al. (1997) reported that synthetic pyrethroid insecticides were less toxic to H. axyridis than to aphids. Insect ecdysone agonists, halofenozide and methoxyfenozide are known to cause premature larval molting, interruption of feeding, and incomplete pupation (Carton et al., 2003). Several terpenoids derived from plants, such as camphor, menthol, catnip, and grapefruit, are known to repel ladybeetles (Riddick et al., 2008). In the case of ladybeetle Hippodamia variegata (Goeze), Almasi et al. (2013) selected pirimicarb and pymetrozine as a low toxic pesticide in contrast to proteus that showed high mortality rate. Rahmani et al. (2013) reported that a new neonicotinoid insecticide, thiamethoxam, decreased the preadult developmental period, while it showed not effect on the adult developmental period. In the case of ladybeetle Imidacloprid and deltamethrin have been reported to be relatively harmful (Bozsik, 2006) to ladybeetle Coccinella septempunctata L., while the two biopesticides Bioshower (a.i. 100% fatty acid) and insecticidal soap (a.i. 20% fatty acids) showed no toxicity (Raudonis et al., 2010). Radha (2013) reported the comparative toxicity of biopesticides and synthetic pesticides against cowpea aphid (Aphis craccivora) and its natural enemy ladybeetle Micrapis discolor. Botanical insecticides (neem seed extracts) and microbial pesticides (spinosad) as were recommended as alternatives to chemical insecticides for the IPM of A. craccivora in cowpea.
In this study, The toxicities of various synthetic insecticides and environment-friendly pesticides to I. koebelei were significantly different (Table 3, 4). In particular, bifenthrin + imidacloprid WP, deltamethrin EC, gamma-cyhalothrin CS, acetamiprid + indoxacarb WP, acetamiprid + etofenprox WP, and acetamiprid WP showed strong toxicity to I. koebelei. Based on the IOBC classification, the three insecticides, bifenthrin + imidacloprid WP, acetamiprid + indoxacarb WP, and acetamiprid + etofenprox WP, were classified as toxicity Class 4 (harmful). Pyriproxyfen EC and spiromesifen SC caused < 20% mortality and are classified as toxicity Class 1 (harmless).
This study showed that I. koebelei is very sensitive to neonicotinoid insecticides. Neonicotinoid insecticides are commonly used against a wide range of herbivorous insect pests such as aphids, mealybugs and whiteflies in greenhouses or farms in Korea (KCPA, 2012). This result is consistent with the results of Lucas et al. (2004) who reported that imidacloprid is highly toxic to both adult and larval stages of ladybeetle Coleomegilla maculata under laboratory conditions. Neonicotinoid insecticides, including proteus, are toxic to both larvae and adults of Coccinellids (Almasi et al., 2013). Bifenthrin + imidacloprid WP, acetamiprid + indoxacarb WP, and acetamiprid + etofenprox WP classified as toxicity Class 4 need to be tested in semi-field or field conditions to determine their effects on mycophagous I. koebelei. Meanwhile, spiromesifen SC, which was placed in Class 1, could be used as a part of the cucumber powdery mildew IPM program in combination with I. koebelei. According to the IOBC, if insecticides were harmless in the laboratory test, it is not necessary to perform further semi-field or field studies (Almasi et al., 2013). However, we suggest that performing further residual toxicity tests may be important for pesticides that showed low contact toxicity to natural enemies in laboratory tests. For example, pyriproxyfen EC, which had low toxicity in the laboratory test, showed markedly high residual toxicity to I. koebelei by decreasing fecundity.
Many environment-friendly pesticides (e.g., Barogaru alpha, Barotok alpha, Daeyou eungjinssak, Eungaetan alpha, Eungsami, Iinsami, Nobug and Suncho) were highly toxic to I. koebei larvae. Daeyou eungjinssak (a.i. natural seed extracts), Eungsami (a.i. Azadirachta indica + Sophora flavescens + microorganism) and Suncho (a.i. Azadirachta indica) were highly toxic to I. koebelei adults and classified as toxicity Class 3 or 4 in the IOBC category. However, Barojin alpha (a.i. Plant extracts) and BT one (a.i. Bacillus thuringiensis) were less toxic to I. koebelei larva. Q pact (a.i. Ampelomyces quisqualis 94013) and Top seed (a.i. Paenibacillus polymyxa AC-1) are microbial fungicides for controlling powdery mildew disease of various agricultural crops (Lee et al., 2004; Kim et al., 2013). The microbial fungicides Q pact, Top seed, and BT one were less toxic to I. koebelei. However, the toxicities of pesticides could differ according to the developmental stages of insects. In this study, I. koebelei larvae were more susceptible to pesticides than adults were. Although BT one did not show residual toxicity to I. koebelei adults, the fecundity of adults from third instar larvae, which had been exposed to BT one, decreased (Table 5, 6). These results show that many botanical or microbial pesticides could decrease the population of I. koebelei.
To date, no study has reported the toxicity of biological or botanical pesticides to mycophagous I. koebelei. We found that many botanical pesticides made from plant extracts or microorganisms could destroy or reduce the population of I. koebelei. The deposit amount of active ingredient on plants can differ according to the formulation or supplement agent of the commercial products. Although, the results of laboratory toxicity test provided some useful information on selective pesticides for I. koebelei, the long-term effect of these pesticides on I. koebelei populations under field conditions could not be explained. Therefore, further field studies regarding these aspects need to be conducted.
Acknowledgments
This study was performed with the support of the cooperative research program for Agricultural Life & Industrial Technology Development (project No. 116089-03-1-SB010), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry, Republic of Korea.
References
-
Almasi, A., Q. Sahahi, K. Talebi, and A. Mardani, (2013), Laboratory evaluation of the toxicity of proteus, pymetrozine, deltamethrin, and pirimicarb on lady beetle Hippodamia Variegata (Goeze) (Col.: Coccinellidae), Journal of Plant Protection Research, 53(2), p143-147.
[https://doi.org/10.2478/jppr-2013-0021]

- Amano, K., (1986), Host range and geographical distribution of the powdery mildew fungi, Tokyo, Japan Scientific Society Press, p741.
- Bhattacharjee, S. S., N. Chakraborty, C. A. Kumar, and A. K. Sahakundu, (1994), Control of the white powdery mildew, Phyllactinia corylea (Pers) Karst, with the ladybird beetle, Illeis indica Timb, (Coccinellidae: Coleoptera), Sericologia, 34, p485-495.
-
Bozsik, A., (2006), Susceptibility of adult Coccinella septempunctata (Coleoptera: Coccinellidae) to insecticides with different modes of action, Pest Manage. Sci, 62(7), p651-654.
[https://doi.org/10.1002/ps.1221]

-
Carton, B., G. Smagghe, and L. Tirry, (2003), Toxicity of two ecdysone agonists, halofenozide and methoxyfenozide, against the multicoloured Asian lady beetle Harmonia axyridis (Col., Coccinellidae), Journal of Applied Entomology, 127(4), p240-242.
[https://doi.org/10.1046/j.1439-0418.2003.00734.x]

-
Cho, J., K. J. Hong, J. K. Yoo, J. R. Bang, and J. O. Lee, (1997), Comparative toxicity of selected insecticides to Aphis citricola, Myzus malisuctus (Homoptera: Aphididae), and the predator Harmonia axyridis (Coleoptera: Coccinellidae), Journal of Economic Entomology, 90, p11-14.
[https://doi.org/10.1093/jee/90.1.11]

-
English-Loeb, G., A. P. Norton, D. Gadoury, R. Seem, and W. Wilcox, (2007), Biological control of grape powdery mildew using mycophagous mites, Plant disease, 91(4), p421-429.
[https://doi.org/10.1094/pdis-91-4-0421]

-
Giorgi, J. A., N. J. Vandenberg, J. V. McHugh, J. A. Forrester, A. ZlipiDski, K. B. Miller, L. R. Shapiro, and M. F. Whiting, (2009), The evolution of food preferences in Coccinellidae, Biol. Control, 51, p215-231.
[https://doi.org/10.1016/j.biocontrol.2009.05.019]

-
Glawe, D. A., (2008), The powdery mildews: a review of the world’s most familiar (yet poorly known) plant pathogens, Annu. Rev. Phytopathol, 46, p27-51.
[https://doi.org/10.1146/annurev.phyto.46.081407.104740]

- Hegazi, M. A., and G. A. El-Kot, (2010), Biological Control of Powdery Mildew on Zinnia (Zinnia elegans, L) Using Some Biocontrol Agents and Plant Extracts, Journal of Agricultural Science, 2, p221-230.
- Hodek, I., H. F. van Emden, and A. Honek, (2012), Ecology and behaviour of the ladybird beetles (Coccinellidae), John Wiley & Sons.
- Kim, J. I., Y. J. Kwon, J. C. Paik, S. M. Lee, S. L. Ahn, H. C. Park, and H. Y. Chu, (1994), Order 23, Coleoptera, In The Entomological Society of Korea and Korean Society of Applied Entomology (ed), Check List of Insects from Korea, Kon-Kuk Univ. Press, Seoul, p117-214.
-
Kim, Y. K., E. J. Choi, S. J. Hong, C. K. Shim, M. J. Kim, H. J. Jee, J. H. Park, E. J. Han, B. K. Jang, and J. C. Yun, (2013), Biological Control of Tomato and Red Pepper Powdery Mildew using Paenibacillus polymyxa CW, The Korean Journal of Pesticide Science, 17(4), p379-387.
[https://doi.org/10.7585/kjps.2013.17.4.379]

- Korea Crop Protection Association, (2012), Guide book of pesticides.
-
Koch, R. L., (2003), The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts, Journal of insect Science, 3(1), p32.
[https://doi.org/10.1093/jis/3.1.32]

- Lee, S. Y, S. B. Lee, Y. K. Kim, and H. K. Kim, (2004), Effect of agrochemicals on mycelial growth and spore germination of a hyperparasite, Ampelomyces quisqualis 94013 for controlling cucumber powdery mildew, The Korean Journal of Pesticide Science, 8(1), p71-78.
-
Lee, S. Y., Y. K. Kim, H. G. Kim, and H. D. Shin, (2007), New hosts of Ampelomyces quisqualis Hyperparasite to powdery mildew in Korea, Res. Plant Dis, 13, p183-190.
[https://doi.org/10.5423/rpd.2007.13.3.183]

- Lin, F. C., C. Y. Lee, C. L. Wang, P. C. Liou, and C. Y. Lin, (2006), Assessment of environmental risk of transgenic papaya ring spot virus resistant papaya on insect and mites, J. Taiwan Agric. Res, 55(4), p222-233.
- Lucas, E., S. Giroux, S. Demougreot, R. M. Coderre, and D. Duchesne, (2004), Compatibility of natural enemy, Coleomegilla maculate lengi (Col., Coccinellidae) and four insecticides used against the Colorado potato beetle (Col., Chrysomelidae), J. Appl. Entomol, 128, p233-239.
- Omkar, Bind R. B., (1996), Record of aphid natural enemies complex of Uttar Pradesh. V. The coccinellids, J. Adv. Zool, 17(1), p44-48.
- Radha, R., (2013), Comparative studies on the effectiveness pesticides for aphid control in cowpea, Research Journal of Agricultural and Forestry Science, 1(6), p1-7.
- Rahmani, S., A. R. Bandani, and Q. Sabahi, (2013), Population statistics and biological traits of Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae) affected by LC30 of thiamethoxam and pirimicarb, Archives of Phytopathology and Plant Protection, 46(15), p1839-1847.
- Raudonis, L., L. Duchovskiene, A. Valiuškaite, and E. Surviliene, (2010), Toxicity of biopesticides to green apple aphid, predatory insects and mite in an apple-tree orchard, Zemdirbyste (Agriculture), 97(1), p49-54.
-
Razdan, V. K., and M. Sabitha, (2009), Integrated disease management: Concepts and practices, In Peshin, R., Dhawan, A.K. eds., Integrated pest management: Innovationdevelopment process, Dordrecht, Springer, p369-389.
[https://doi.org/10.1007/978-1-4020-8992-3_15]

- Recuenco-Adorada, J. D., and V. P. Gapud, (1998), Philippine species of Illeis mulsant (Coleoptera: Coccinellidae: Coccinellinae: Psylloborini), The Philippine Entomologist, 12(1), p43-53.
- Riddick, E. W., A. E. Brown, and K. R. A. E., (2008), Harmonia axyridis adults avoid catnip and grapefruitderived terpenoids in laboratory bioassays, Bulletin of Insectology, 61(1), p81.
-
Romero, D., A. de Vicente, H. Zeriouh, F. M. Cazorla, D. Fernández-Ortuño, J. A. Torés, and A. Pérez-García, (2007), Evaluation of biological control agents for managing cucurbit powdery mildew on greenhouse-grown melon, Plant Pathology, 56, p976-986.
[https://doi.org/10.1111/j.1365-3059.2007.01684.x]

- SAS Institute, (2008), SAS Online Doc(r) 9.2. SAS Institute, Cary, NC.
- Sasaji, H., (1998), Natural history of the ladybirds, University of Tokyo Press, p251.
- Segarra, G., M. Reis, E. Casanova, and M. I. Trillas, (2009), Control of powdery mildew (Erysiphe polygoni) in tomato by foliar applications of compost tea, Journal of Plant Pathology, 91, p683-689.
-
Sutherland, A. M., and M. P. Parrella, (2009), Mycophagy in Coccinellidae: review and synthesis, Biological Control, 51, p284-293.
[https://doi.org/10.1016/j.biocontrol.2009.05.012]

-
Tabata, J., C. M. D. Moraes, and M. C. Mescher, (2011), Olfactory cues from plants infected by powdery mildew guide foraging by a mycophagous ladybird beetle, PLoS ONE (www.plosone.org), 6(8), pe23799.
[https://doi.org/10.1371/journal.pone.0023799]

-
Takeuchi, M., Y. Sasaki, C. Sato, S. Iwakuma, A. Isozaki, M. Tamura, (2000), Seasonal host utilization of mycophagous ladybird Illeis koebelei (Coccinellidae: Coleoptera), Japanese Journal of Applied Entomology and Zoology, 44, p89-94.
[https://doi.org/10.1303/jjaez.2000.89]

- Tanaka, K., S. Endo, and H. Kanano, (2000), Toxicity of insecticides to predators of rice plant hoppers: spider, the mirid bug and dryinid wasp, Japanese Society of Applied Entomology and Zoology, 35(1), p177-187.
- Wu, W, D. Liu, P. Zhang, and Z. Zhang, (2011), Community structure and diversity of ladybugs in Baihualing of Gaoligong Mountain I, Plant Diseases and Pests, 2(4), p46-48.