Developmental Toxic Effects of Phosmet on Zebrafish (Danio rerio) Embryos
Abstract
Phosmet, an organophosphate pesticide, has been widely applied to plants and animals. As the adverse effects of phosmet on the aquatic vertebrates are not fully understood, the present study was aimed to evaluate the developmental toxicity of phosmet using zebrafish (ZF) (Danio rerio) embryos. The ZF embryos were treated with 2.96, 4.44, 6.66, 10, and 15 mg/L of phosmet. Consequently, the embryo mortalities and developmental deformities were recorded at 24, 48, 72, and 96 hours post fertilization (hpf). The results showed that the effects of phosmet on ZF embryos were time- and dose-dependent. The median lethal concentration (LC50) of phosmet at 96 h was 7.95 ± 0.30 mg/L. The results of developmental toxicity showed that the phosmet induced a series of deformities in somites, tails, spines, yolk-sacs, hearts and swim bladders of ZF embryos and larvae. Among the deformities, the heart- and growth-related deformities were the most significant. Further, the phosmet-treated ZF displayed an abnormal touch-evoked response and swimming, suggesting potential neurodevelopmental toxicity of phosmet. The median effective concentration (EC50) of phosmet at 96 h was 4.38 ± 0.18 mg/L and the 96 h teratogenic index (TI) value was 1.7. Overall, these results indicate that phosmet is a teratogen and severely affects the growth of ZF in the earlier developmental stages.
Keywords:
Acute toxicity, Phosmet, Teratogen, Zebrafish embryosIntroduction
Organophosphates (OPs) account for around 50% of the globally used chemical pesticides. Although these compounds are usually degraded faster, their presence and effects may be higher than expected in the environment as OPs may need to be used more often and more frequently to control pests. OPs act as a competitive inhibitor of pseudocholinesterase and acetylcholine esterase (AChE) and contribute to pathological excess of acetylcholine (ACh) in the body by preventing the hydrolysis ACh (Laetz et al., 2020). Excessive accumulation of ACh is linked to cardiotoxicity, neurotoxicity, mortality and swimming performance of fish (Qayoom et al., 2014).
Phosmet is one of the most commonly used OP pesticides on the plants and animals for controlling colding moth, aphids, mites, suckers, and fruit flies (US EPA, 2006). Wide usage of phosmet may lead to the accumulation in the water bodies and appears to present adverse effects to freshwater vertebrates (Mitra and Maitra, 2018). Acute exposure studies of phosmet conducted on animals showed that the phosmet is highly toxic through skin and inhalation (US EPA, 2006). Phosmet also showed adverse effects on normal development and ossification of mice. (Bleyl, 1980). According to the available acute toxicity data, phosmet is toxic to Daphnia magna (48 h EC50: 0.0056 mg/L) and other fish such as Oncorhynchus mykiss (96 h LC50: 0.241 mg/L) and Lepomis macrochirus (96 h LC50: 0.07 mg/L) (European chemical agency, 2008). Recently, a study conducted on juvenile Oncorhynchus kisutch showed that phosmet can also inhibit the AChE (Laetz et al., 2020).
The ZF has been suggested as the best option for acute toxicity studies (Nagel, 2002; Lammer et al., 2009) and is widely used in the assessment of the environmental risk of pesticides and other chemical substances (Lammer et al., 2009). Due to its small size (2-4 cm), high fertility, large brood size and short life cycle, ZF is regarded as an excellent alternative to the animal models (Lammer et al., 2009). Also, ZF embryos are transparent, and embryonic stages as well as organogenesis are well established (Kimmel et al., 1995). Therefore, it is possible to identify and predict a range of morphological and behavioral adverse effects of the drug in ZF's toxicity model (Busquet et al., 2014).
The toxic effects of phosmet on fish have not been studied well. This study was therefore designed to assess the teratogenic potential of phosmet with ZF embryos. The current study was performed following Organization for Economic Cooperation and Development (OECD) guideline 236 to obtain a dose-related response of ZF embryos to phosmet.
Materials and methods
Materials
Phosmet, [2-(Dimethoxyphosphinothioylthiomethyl) isoindoline- 1,3-dione] (purity 98%) and 2,4-dichloroaniline (purity 98%) were obtained from Sigma Aldrich (St. Louis, MO, USA). The stock solution was prepared in acetone and the aliquots were stored at -20oC in the dark condition. The test solution was freshly prepared by dissolving required quantities of stock solution to the E3 medium (29 mg of NaCl, 0.83 mg of KCl, 4.8 mg of CaCl2, 8.15 mg of MgCl2, and 10 μl of 1% methylene blue per 100 ml of deionized water, pH 7.2). All the chemicals used in this study were obtained from Sigma Aldrich unless otherwise stated.
Zebrafish maintenance and embryo collection
ZF were maintained in 50 L glass aquariums filled with dechlorinated tap water and equipped with continuous aeration and filtration systems. The fish batches were kept under a constant temperature of 25 ± 1oC and 14 h light/ 10 h dark photo period. The ZF were fed two to three times a day with fish flakes (Top meal, Tabia, Korea), live brine shrimp (artemia (INVE aquaculture, Dendermonde, Belgium)) and blood worms (Hikari Bio-pure, USA).
Mass spawning was performed for embryo collection. Briefly, five males and five females were transferred into a nylon mesh (30×20×13 cm) which was installed in a plastic aquarium (40×25×18 cm, Daehan Biolink, Korea) contained 10 L of dechlorinated tap water. The fish were maintained at 14 h light/10 h dark regime and temperature of 26.5-27.0oC. The fertilized eggs were collected on the next day and were washed 5 times in the E3 medium.
Toxicity assay
The assay was performed according to OECD guideline 236 (Fish Embryo Acute Toxicity Test (FET)) (OECD, 2013) in triplicate. Briefly, the embryos were incubated with various doses of phosmet. For the main assay, 24 well plates (SPL life sciences, Korea) were used. Five test doses of phosmet (2.96, 4.44, 6.66, 10 and 15 mg/L), positive control (4 mg/L of 2,4-dichloroaniline), and control (0.05% of acetone) were used. In each plate, 20 wells (2 ml/well) were filed with respective test solutions and the other four wells (2 ml/well) were filled with E3 medium (internal controls). The embryos were dropped into each well and the plates were transferred to an incubator to maintain 26±1oC and the dark conditions. After 24 hrs, from each well, ~1.5 ml of test solution was replaced with freshly prepared test chemicals, respectively.
Morphological abnormalities scoring
Mortality and deformities of the ZF embryos were scored under a stereomicroscope (Stemi 508, Zeiss, Germany) at 24, 48, 72, and 96 hpf. The embryos that showed coagulation or failed to succeed the normal development were considered as dead. The defects in the somites and tail detachment were scored at 24 hpf. Abnormalities for tail morphology, tail blood flow, and eye pigmentation were scored at 48 hpf. Yolk-sac edema, pericardial edema, and unhatched embryos were counted at 72 hpf. At 96 hpf, kink formation in the tail, fish that showed side-wise position (usually ZF “float up” at 96 hpf), swimming ability in response to touch, unhatched embryos, and spine deformities were scored.
The deformity scoring and analysis were performed as reported earlier (Slederslaghs et al., 2009). The deformities score was given in a binominal way (normal (0) or abnormal (1)). The percentages of embryo mortality was calculated as the ratio of dead embryo/larvae at the time point of observation over the number of embryos at the start of the exposure (20 fertilized eggs). The percentage of deformity at 24 and 48 hpf was calculated as the ratio of embryos that showed deformity over the number of live embryos at the time point. The percentage of deformity at 72 and 96 hpf was calculated as the ratio of embryos that showed deformity over all embryos at the time point. The resulting percentage data from three independent experiments (n=3), each with 20 replicates per concentration, were used to calculate the LC50 and EC50 values. For the 48 h EC50 calculation, deformities such as abnormal somites, tail detachment, abnormal tail morphology, abnormal eye pigmentation, hyperemia, and abnormal tail blood flow were used. For the 96 h EC50 calculation, all scored deformities were used.
Heartbeat count
The heartbeats were counted for 20 seconds under the microscope after 48 hrs of phosmet treatment. The data were normalized to heartbeats per minute and presented.
Body length
To measure the body length, the fish treated with phosmet for 96 hrs were anesthetized with a final concentration of 0.1% ethyl 3-aminobenzoate methanesulfonate (Sigma, St.Louis, USA) in 24 well plates. Photographs were captured under a stereomicroscope and the length of the fish (mouth tip to end of the tail fin) was obtained using OptiView 3.7 software (Korealabtech, Seongnam, Korea).
Data analysis
GraphPad Prism software version 5.0 (GraphPad Software, USA) was used to analyze the LC50 values, EC50 values, and other statistical analyses. Data were presented as mean ± SEM. One-way analysis of variance (Kruskal- Wallis method) was applied to determine the effect of concentration. Unpaired t-test was used to calculate statistical significance between control and other phosmet treatments. The p values ≤0.05 were considered as statistically significant. The teratogenic index (TI) was calculated by dividing the LC50 by the EC50 (LC50/EC50).
Results and Discussion
Acute toxicity of phosmet
Phosmet-induced toxicity was time- and dose-dependent. Whereas there was no embryonic mortality found in the internal controls until 96 hpf, ~ 85% of embryo mortality was found in 4-dichloroaniline treatment within 24 hpf. These observations indicate that the obtained experimental data were reliable. No mortalities were found in the 0.05% acetone-treated control group until 96 hpf (Table 1). The cumulative mortality percentage (96 hpf) of 2.96, 4.44, 6.66, 10, and 15 mg/L phosmet-treated groups were noted as 6.7 ± 1.7, 21.7 ± 1.7, 36.7 ± 3.3, 65.0 ± 2.9, and 98.3 ± 1.7 respectively. The 48 h and 96 h LC50 of phosmet were 8.40 ± 0.68 and 7.95 ± 0.30 mg/L, respectively (Table 2).
Percentage of deformities (3 independent experiments) observed at 24, 48, 72, and 96 hrs after phosmet treatment
Developmental toxicity of phosmet
Acetone-treated embryo/larvae displayed normal growth and phenotype during experimental periods (Table 1). However, embryo/larvae treated with phosmet exhibited several developmental deformities from a dose of 2.96 mg/ L. All deformities were increased in a dose-dependent manner. The summarized data on several deformities observed at 24, 48, 72, and 96 hpf of phosmet-treatment are shown in Table 1. Among the deformities, cardiac- and growth-related deformities were the most common (Table 1 and Fig. 1). Table 2 shows the 48 h and 96 h EC50 values of phosmet. The ratio of cumulative LC50 values to cumulative EC50 values was greater than 1 (Table 2), indicating that phosmet is a teratogen to ZF (Slederslaghs et al., 2009).
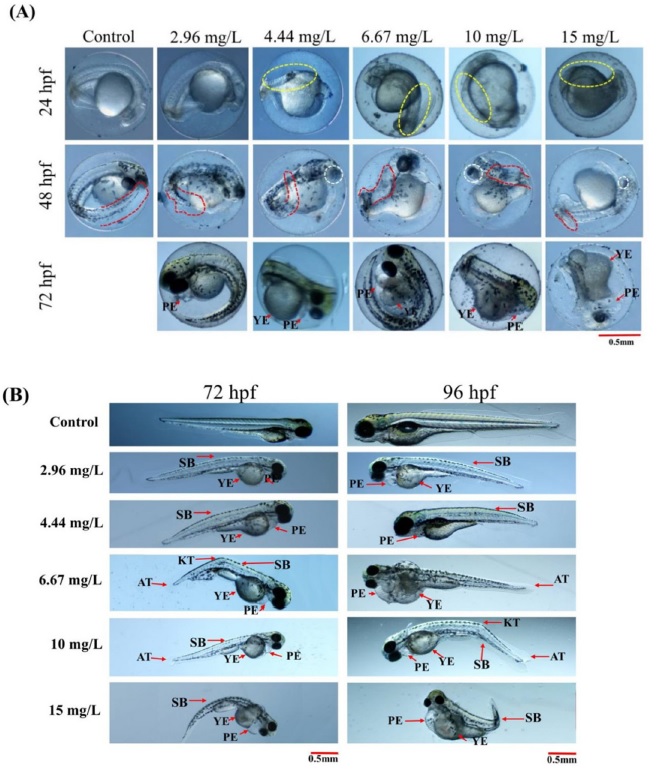
Representative images showing phosmet-induced deformities at (A) 24 hpf, 48 hpf and 72 hpf (unhatched embryos) (B) 72 hpf and 96 hpf. Controls showed normal morphology until 96 hpf. Yellow dotted circle, somites; Red dotted circle, shape of the tail; white dotted circle, eyes; PE, pericardial edema; YE, yolk-sac edema; KT, kink in the tail; AT, abnormal tail; SB, spine bending. Scale 0.5 mm.
Fig. 1 shows the representative images of phosmet-induced deformities and the relevant descriptions. After 24 hrs of phosmet treatment, tail detachment and somite formation were quantified. There was no significant effect of phosmet on the tail detachment of ZF embryos (Table 1). However, the somite formation was significantly disrupted in phosmet-treated groups (p ≤ 0.005). The embryos that were treated from a dose of 4.44 mg/L of phosmet showed irregularly placed somite boundaries (Table 1 and Fig. 1A). At this dose, 57.0 ± 2.2% of ZF embryos displayed abnormal somite phenotype.
Delayed retina pigmentation was one of the most significant deformities in phosmet-treated ZF embryos at 48 hpf (p ≤ 0.05). The ZF embryos treated with 2.96 mg/L of phosmet showed normal eye pigmentation as controls (Table 1). However, the ZF embryos treated from a dose of 4.44 mg/L of phosmet showed delayed eye pigmentation. Abnormal tail morphology (AT) was another significantly induced deformities in phosmet-treated embryos at 48 hpf (p ≤ 0.005). AT was observed from the lowest dose 2.96 mg/L of phosmet in a dose-dependent manner (Table 1).
Most of the phosmet-treated embryos were not hatched (p ≤ 0.01 at 72 hpf and p ≤ 0.006 at 96 hpf). This effect was observed from a dose of 4.44 mg/L of phosmet and more than 90% of embryos were not hatched at doses of 10 and 15 mg/L phosmet. Consistent with these findings, there was a significant decrease in the hatching rate of ZF embryos after treatment with OP pesticides (Sisman, 2010; Rahman et al., 2020). Reduced hatchability might be due to disturbances in the secretion of proteases which are required for chorion digestion or paralysis caused by neurotoxicity (Schoots et al., 1982; Vittozzi et al., 2001; Qayoom et al., 2014).
Yolk-sac edemas (YEs) were another prominent deformities in phosmet-treated groups (p ≤ 0.005). YEs were evident from the lowest dose of 2.96 mg/L of phosmet and it was dose-dependent (Table 1 and Fig. 2B).
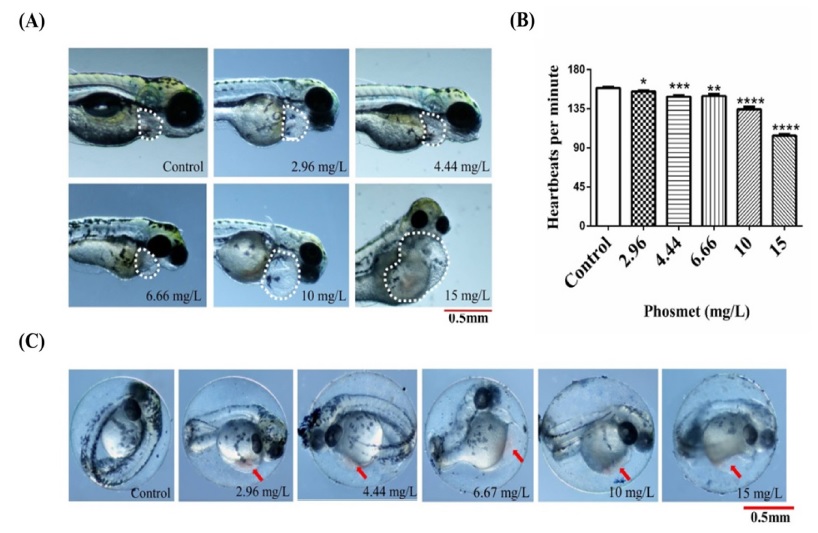
Effects of phosmet on the heart development and function. (A) Representative images of ZF embryos showing pericardial edema at the indicated dose of phosmet. The dotted white line indicates the morphology of the cardiac region. (B) Heart beats per minute at designated doses of phosmet at 48 hpf. The results are mean ± SEM (n=15 embryos). *(p ≤ 0.05), **(p ≤ 0.005), ***(p ≤ 0.001), ****(p < 0.0001). (C) Representative images showing hyperemia (arrowhead) at indicated doses.
Tail kinks (KT) (p ≤ 0.001), spine bending (p ≤ 0.001) and abnormal tail morphology (AT) (p ≤ 0.005) were other types of deformities observed after phosmet treatment. All these deformities were observed from the lowest dose of phosmet (2.96mg/L). Similar deformities were observed in ZF embryos after treated with OP pesticides such as diazinon and sumithion (Osterauer and Köhler, 2008).
Effect of phosmet on cardiovascular development and function
The ZF heart appears to be a primary target for developmental toxicity (Hill et al., 2005) as it is one of the first organs to develop and function. Aiming to understand the phosmet-induced cardiac-toxicity (Yalcin et al., 2017), heartbeats, regional blood flow and pericardial edema formation were assessed both in the control and phosmet-treated groups. Cardiovascular deformities were increased in a dose-dependent manner (Table 1 and Fig. 2). Phosmet induced pericardial edema (PE) in a dose-dependent manner (p ≤ 0.01). The high incidence of PE was found in all the doses of phosmet treatment (72 hpf, Table 1) and Fig. 2A), suggesting that phosmet interferes with cardiogenesis of ZF.
To investigate the effect of phosmet on cardiovascular function, the heartbeats of the ZF embryos in control and phosmet treatment groups were counted. The average heartbeats of the ZF embryos treated with various doses of phosmet at 48 hpf are shown in Fig. 2B. Phosmet showed a dose-dependent decrease in the heartbeats (p ≤ 0.0001). The significant effect of phosmet on the heartbeats was observed from 2.96 mg/L. The average heartbeats per minute in the controls were 158.8 and in phosmet-treated groups, the average heartbeats per minute were significantly reduced (154.8-104.0) compared to controls, indicating that the phosmet affects the cardiac function of ZF.
There was a high incidence of hyperemia in phosmettreated groups when compared to the control group (p ≤ 0.001). Phosmet significantly induced hyperemia from the lowest dose of 2.96 mg/L ((Table 1 and Fig. 2C), suggesting an improper blood flow. To further understand the circulatory defects, the blood flow at the dorsal aorta region was observed. Phosmet significantly affected the blood flow of ZF (p ≤ 0.005). Whereas the blood flow of the acetone-treated embryos was normal, the blood flow of embryos treated with phosmet was either slow or completely absent ((Table 1) and images not shown), further supporting a notion that phosmet affects cardiovascular function.
In ZF, PE is considered as an indicator of abnormal cardiac development, and heartbeat is considered an important cardiac function indicator (Gut et al., 2017). Several cardiac-related deformities (PE, reduced heartbeat, hyperemia, and abnormal blood flow) were observed in ZF embryos/larvae after exposure to phosmet ((Table 1), Fig. 1 and Fig. 2). Similar deformities were documented in ZF embryo/larvae exposed to OP pesticides such as diazinon, (Osterauer and Köhler, 2008; Cao et al., 2018) dichlorvos, (Sisman, 2010) and sumithion (Rahman et al., 2020). Earlier reports showed that the OPs caused over stimulation of acetylcholine receptors (Qayoom et al., 2014; Laetz et al., 2020). Hsieh and Liao reported that overexpression of muscarinic acetylcholine receptors (mAChRs) led to bradycardia (reduced heart rate) in ZF (Hsieh and Liao, 2002). And Steele et al., observed that inhibition of mAChRs attenuated hypoxic bradycardia in ZF (Steele et al., 2009). Thus, perturbations of muscarinic signaling might be one of the possible explanation for the phosmetinduced abnormal cardiac function of the ZF. However, further experiments are required to know the exact cause of phosmet induced cardiac toxicity.
Effect of phosmet on growth and behavior
The body length measurement showed that the phosmet significantly inhibited the overall growth of the ZF (p ≤ 0.001). The average body lengths of phosmet-treated groups were reduced in a dose-dependent manner (Fig. 3). The average body lengths of controls were 4.17 mm. The average body lengths of phosmet-treated fish were 3.45 (2.96 mg/L), 3.40 (4.44 mg/L), 3.22 (6.66 mg/L), 3.07 (10 mg/L), and 2.70 (15 mg/L) mm, respectively.
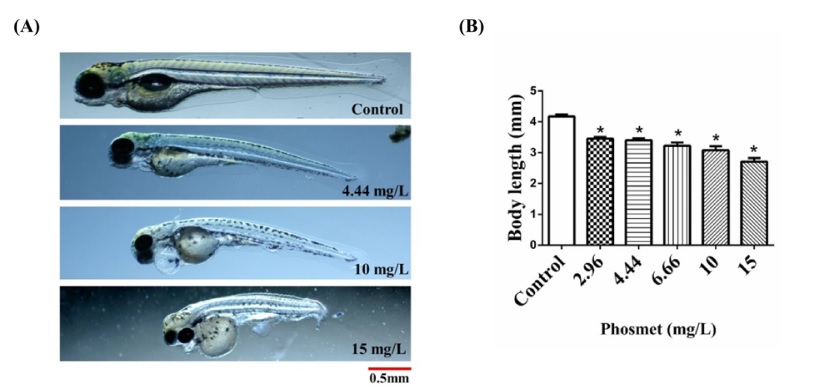
Effects of phosmet on the growth of ZF. (A) Representative images of ZF larvae without treatment and treated with 4.44, 10, and 15 mg/L of phosmet at 96 hpf (B) Average body length of ZF larvae at indicated doses of phosmet at 96 hpf. The results are mean ± SEM (n=15 embryos). *(p < 0.0001).
Phosmet treatment significantly affected swim bladder formation (p ≤ 0.01) and showed a ‘side-wise position’ (p ≤ 0.05). Whereas the controls presented normal swim bladders, phosmet-treated ZF showed either malformed or uninflated swim bladders (Table 1). The swim bladder, the gas-filled organ, is essential for the buoyancy regulation for most of the teleost species (Finney et al., 2006).
The behavioral defects of ZF larvae have been considered as sensitive endpoints for estimating neurotoxicity (Selderslaghs et al., 2010; Palmer et al., 2017). The phosmet significantly interfered with the touch-evoked response (p ≤ 0.005) and swimming behavior (p ≤ 0.005) of the ZF. The control and 2.96 mg/L of phosmet-treated groups responded well to touch stimuli and showed a normal swimming behavior (Table 1). However, the 4.44-15 mg/L of phosmet-treated groups showed mild~no response to touch stimuli and exhibited swimming disabilities. These results imply the potential neurodevelopmental toxicity of phosmet. ZF exposed to endosulfan (Pereira et al., 2012), chlorpyrifos (Tilton et al., 2011), and profenofos (Pamanji et al., 2015) also showed swimming disabilities.
OPs act as a nervous system poisons by inhibiting AChE (Fulton and Key, 2001). AChE inhibition leads to sublethal neurotoxicity and disrupts the normal swimming behavior of the fish (Sandahl et al., 2005; Tierney et al., 2007). OPs-mediated inhibition of AChE leads to the accumulation of ACh in the nervous termination. This, in turn, triggers overstimulation of nicotinic acetylcholine receptors (nAChRs) and mAChRs, key regulators of cellular signaling, neuronal signaling, learning and memory formation (Vittozzi et al., 2001; Braida et al., 2014; Park et al., 2016; Pedersen et al., 2018; Vasamsetti et al., 2019 ). Recently, it was reported that phosmet produced a concentrationdependent inhibition of AChE in fish (Laetz et al., 2020) and AChE was critical for the normal neuronal and muscular development of ZF (Behra et al., 2002). Thus, it is plausible that the phosmet-induced behavioral defects observed in ZF might be the consequences of AChE inhibition. It is also possible that phosmet-induced deformities like uninflated swim bladders, abnormal pectoral fins and curved spines could be other possible reasons for abnormal swimming (Müller and van Leeuwen, 2004). Thus, additional works are required to find out the exact cause of phosmetinduced swimming defects.
To summarize, these results demonstrated that phosmet exposure at early life stages disturbed the normal growth and development of the ZF. Phosmet induced several developmental deformities at a dose significantly lower than the lethal dose, indicating that phosmet is a teratogen. Further investigations are required to gain deeper mechanistic insights into phosmet-induced toxicity.
Acknowledgments
This research was supported by a grant from the Research Program for Agriculture Science and Technology Development (Project No. PJ01423702), National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea.
Disclosure of potential conflicts of interests
The authors declare that they have no conflict of interest.
References
-
Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, et al., 2002. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 5(2):111-118.
[https://doi.org/10.1038/nn788]

- Bleyl DW, 1980. Embryotoxicity and teratogenicity of phosmet in mice. Arch. Exp. Veterinarmed. 34 (5):791-795.
-
Braida D, Ponzoni L, Martucci R, Sparatore F, Gotti C, et al., 2014. Role of neuronal nicotinic acetylcholine receptors (nAChRs) on learning and memory in zebrafish. Psychopharmacol. 231(9):1975-1985.
[https://doi.org/10.1007/s00213-013-3340-1]

-
Busquet F, Strecker R, Rawlings JM, Belanger SE, Braunbeck T, et al., 2014. OECD validation study to assess intra-and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. 69(3):496-511.
[https://doi.org/10.1016/j.yrtph.2014.05.018]

-
Cao F, Souders II CL, Li P, Pang S, Qiu L, et al., 2018. Biological impacts of organophosphates chlorpyrifos and diazinon on development, mitochondrial bioenergetics, and locomotor activity in zebrafish (Danio rerio). Neurotoxicol. Teratol. 70:18-27.
[https://doi.org/10.1016/j.ntt.2018.10.001]

- European chemical agency, 2008. CLH report for phosmet. https://echa.europa.eu/documents/10162/c8107329-a056-5b29-27bb-574562ba2e45, (Accessed Sep.10 2020).
-
Finney JL, Robertson GN, McGee CA, Smith FM, Croll RP, 2006. Structure and autonomic innervation of the swim bladder in the zebrafish (Danio rerio). J. Comp. Neurol. 495(5):587-606.
[https://doi.org/10.1002/cne.20948]

-
Fulton MH, Key PB, 2001. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ. Toxicol. Chem. 20(1):37-45.
[https://doi.org/10.1002/etc.5620200104]

-
Gut P, Reischauer S, Stainier DY, Arnaout R, 2017. Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 97(3):889-938.
[https://doi.org/10.1152/physrev.00038.2016]

-
Hill AJ, Teraoka H, Heideman W, Peterson RE, 2005. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 86(1):6-19.
[https://doi.org/10.1093/toxsci/kfi110]

-
Hsieh DJY, Liao CF, 2002. Zebrafish M2 muscarinic acetylcholine receptor: cloning, pharmacological characterization, expression patterns and roles in embryonic bradycardia. Br. J. Pharmacol. 137(6):782-792.
[https://doi.org/10.1038/sj.bjp.0704930]

-
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, 1995. Stages of embryonic development of the zebrafish. Dev. Dyn. 203(3):253-310.
[https://doi.org/10.1002/aja.1002030302]

-
Laetz CA, Baldwin DH, Scholz NL, 2020. Sublethal neurotoxicity of organophosphate insecticides to juvenile coho salmon. Aquat. Toxicol. 221:105424.
[https://doi.org/10.1016/j.aquatox.2020.105424]

-
Lammer E, Carr G, Wendler K, Rawlings J, Belanger S, et al, 2009. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Phys. C. 149(2):196- 209.
[https://doi.org/10.1016/j.cbpc.2008.11.006]

- Mitra A, Maitra S, 2018. Reproductive toxicity of organophosphate pesticides. Ann. Clin. Toxicol. 2018; 1(1) 1004. 1-8.
-
Müller UK, van Leeuwen JL, 2004. Swimming of larval zebrafish: ontogeny of body waves and implications for locomotory development. J. Exp. Biol. 207(5):853-868.
[https://doi.org/10.1242/jeb.00821]

- Nagel R, 2002. DarT: The embryo test with the Zebrafish (Danio rerio) a general model in ecotoxicology and toxicology. Altex 19(1):38-48.
- OECD, 2013. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, Guidelines for the Testing of Chemicals.
-
Osterauer R, Köhler H-R, 2008. Temperature-dependent effects of the pesticides thiacloprid and diazinon on the embryonic development of zebrafish (Danio rerio). Aquat. Toxicol. 86(4):485-494.
[https://doi.org/10.1016/j.aquatox.2007.12.013]

-
Palmer T, Fredrik EK, Enqvist O, Olsson R, Astrom K, et al, 2017. Action sequencing in the spontaneous swimming behavior of zebrafish larvae - implications for drug development. Sci. Rep. 7(1): DOI: 10.1038/s41598-017-03144-7
[https://doi.org/10.1038/s41598-017-03144-7]

-
Pamanji R, Yashwanth B, Bethu M, Leelavathi S, Ravinder K, et al, 2015. Toxicity effects of profenofos on embryonic and larval development of Zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 39(2):887-897.
[https://doi.org/10.1016/j.etap.2015.02.020]

-
Park YS, Liu Z, Vasamsetti BMK, Cho NJ, 2016. The ERK1/2 and mTORC1 Signaling Pathways Are Involved in the Muscarinic Acetylcholine Receptor‐Mediated Proliferation of SNU‐407 Colon Cancer Cells. J. Cell. Biochem. 117(12): 2854-2863.
[https://doi.org/10.1002/jcb.25597]

-
Pedersen JE, Bergqvist CA, Larhammar D, 2018. Evolution of the muscarinic acetylcholine receptors in vertebrates. Eneuro 5(5).
[https://doi.org/10.1523/ENEURO.0340-18.2018]

-
Pereira VM, Bortolotto JW, Kist LW, Azeedo B, Fritsch RS, et al., 2012. Endosulfan exposure inhibits brain AChE activity and impairs swimming performance in adult zebrafish (Danio rerio). Neurotoxicology. 33(3):469-475.
[https://doi.org/10.1016/j.neuro.2012.03.005]

- Qayoom I, Balkhi M, Mukhtar M, Bhat FA, Shah FA, 2014. Biochemical toxicity of organophosphate compounds in fishes. Skuast. J. 16(1):1-13.
-
Rahman MS, Islam SM, Haque A, Shahjahan M, 2020. Toxicity of the organophosphate insecticide sumithion to embryo and larvae of zebrafish. Toxicol. Rep. 7:317-323.
[https://doi.org/10.1016/j.toxrep.2020.02.004]

-
Sandahl JF, Baldwin DH, Jenkins JJ, Scholz NL, 2005. Comparative thresholds for acetylcholinesterase inhibition and behavioral impairment in coho salmon exposed to chlorpyrifos. Environ. Toxicol. Chem. 24(1):136-145.
[https://doi.org/10.1897/04-195R.1]

-
Schoots AF, Stikkelbroeck JJ, Bekhuis JF, Denucé JM, 1982. Hatching in teleostean fishes: fine structural changes in the egg envelope during enzymatic breakdown in vivo and in vitro. J. Ultrastruct. Res. 80(2):185-196.
[https://doi.org/10.1016/S0022-5320(82)90017-X]

-
Selderslaghs IW, Rompay AR, Coen WD, Witters HE, 2009. Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod. Toxical. 28(3):308-320.
[https://doi.org/10.1016/j.reprotox.2009.05.004]

-
Selderslaghs IW, Hooyberghs J, De Coen W, Witters HE, 2010. Locomotor activity in zebrafish embryos: a new method to assess developmental neurotoxicity. Neurotoxicol. Teratol. 32(4):460-471.
[https://doi.org/10.1016/j.ntt.2010.03.002]

-
Şişman T, 2010. Dichlorvos-induced developmental toxicity in zebrafish. Toxicol. Ind. Health. 26(9):567-573.
[https://doi.org/10.1177/0748233710373089]

-
Steele SL, Lo KHA, Li VWT, Cheng SH, Ekker M, et al., 2009. Loss of M2 muscarinic receptor function inhibits development of hypoxic bradycardia and alters cardiac β- adrenergic sensitivity in larval zebrafish (Danio rerio). Am. J. Physiol. 297(2):R412-R420.
[https://doi.org/10.1152/ajpregu.00036.2009]

-
Tierney K, Casselman M, Takeda S, Farrell T, Kennedy C, 2007. The relationship between cholinesterase inhibition and two types of swimming performance in chlorpyrifos‐exposed coho salmon (Oncorhynchus kisutch). Environ. Toxicol. Chem. 26(5):998-1004.
[https://doi.org/10.1897/06-459R.1]

-
Tilton FA, Bammler TK, Gallagher EP, 2011. Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately, or as mixtures. Comp. Biochem. Phys. C. 153(1):9-16.
[https://doi.org/10.1016/j.cbpc.2010.07.008]

- US EPA, 2006. Reregistration eligibility decision, Phosmet. https://archive.epa.gov/pesticides/reregistration/web/pdf/phosmet_red.pdf, (Accessed Mar. 21. 2020).
-
Vasamsetti BMK, Liu Z, Park YS, Cho NJ, 2019. Muscarinic acetylcholine receptors regulate the dephosphorylation of eukaryotic translation elongation factor 2 in SNU-407 colon cancer cells. Biochem. Biophys. Res. 516(2):424-429.
[https://doi.org/10.1016/j.bbrc.2019.06.059]

-
Vittozzi L, Fabrizi L, Di Consiglio E, Testai E, 2001. Mechanistic aspects of organophosphorothionate toxicity in fish and humans. Environ. Int. 26(3):125-129.
[https://doi.org/10.1016/S0160-4120(00)00102-1]

-
Yalcin HC, Amindari A, Butcher JT, Althani A, Yacoub M, 2017. Heart function and hemodynamic analysis for zebrafish embryos. Dev. Dyn. 246(11):868-880.
[https://doi.org/10.1002/dvdy.24497]

Bala Murali Krishana Vasamsetti, Department of Agrofood Safety and Crop Protection, National Institute of Agricultural Sciences, RDA, Postdoctoral researcher, designed and conducted the experiments, analyzed the data and wrote the manuscript. https://orcid.org/0000-0001-5529-418X
Nam Seok Kim, Department of Agro-food Safety and Crop Protection, National Institute of Agricultural Sciences, RDA, Research assistant, performed experiments. https://orcid.org/0000-0003-4046-1744
Kyongmi Chon, Department of Agro-food Safety and Crop Protection, National Institute of Agricultural Sciences, Doctor of Philosophy, Fund acquisition, designed the experiments and revised the manuscript. https://orcid.org/0000-0003-2143-2614
Hong-Hyun Park, Department of Agro-food Safety and Crop Protection, National Institute of Agricultural Sciences, RDA, Doctor of Philosophy, designed the experiments. https://orcid.org/0000-0003-1213-0665
