Efficacy of Nematicides Against Two Destructive Nematodes; Helicotylenchus microlobus (Nematoda: Tylenchida) and Mesocriconema nebraskense (Nematoda: Criconematina) in Turfgrass
Abstract
The two plant-parasitic nematodes, Helicotylenchus microlobus (spiral nematode) and Mesocriconema nebraskense (ring nematode) widely occur in Korean turfgrass fields. Control and management of these nematodes including other turfgrass nematodes is still at the basic level and therefore, a reliable control strategy is much needed to safeguard the golf courses from plant-parasitic nematodes. In this study, laboratory and field experiments were carried out to test the efficacy of four different nematicides against spiral and ring nematodes. Fluopyram showed a high control efficacy with LC50 of as low as 0.01440 ppm and LC90 of 17.241 ppm after 72 hours of treatment. There was a strong, and moderate linear correlation between the tested doses of abamectin and the corrected mortalities of H. microlobus and M. nebraskense, respectively. However, fosthiazate showed the greatest efficacy among all the tested nematicides in field conditions. This study therefore, suggests that these nematicides could be used in the control of Mesocriconema nebraskense and Helicotylenchus microlobus in turfgrass fields.
Keywords:
Acute toxicity, chemical control, ring nematode, spiral nematode, turfgrassIntroduction
During the last few decades, the turfgrass industry has become one of the significant economic growth promoters worldwide, especially as a resource in creating surfaces for outdoor sports, recreational activities and providing aesthetic beauty (Vandenbossche et al., 2011). Turfgrass production has become one of the important economic agricultural businesses in many countries. Turfgrass in Korea is mostly used not only in creation of outdoor sports and recreational surfaces but also as lawn cover in home gardens, institutions, and cemeteries (Kim, 1991; Kang et al., 2016). Like other countries, Korean golf industry continues to grow, and is worth an estimated 11.4 trillion won (Bae et al., 2013; Lee et al., 2014; Koreabizwire, 2017). However, even with the increasing economic importance and demand for turfgrass in Korea, its cultivation methods, and pest and disease management systems are far from being optimal in the country (Choo et al. 2017). More specifically, the knowledge on occurrence, damage potential, and control of important turfgrass nematodes is very insufficient for a better turfgrass management system. Moreover, keeping a healthy golf course, especially putting greens un-attacked by various insect pests including nematodes is the most challenging job, yet it has a bearing on the golf course usability, and may influence golfers’ choice among the available golf courses (Crow, 2005; 2007; Brandenburg and Freeman, 2012). Various plant-parasitic nematodes are considered to be a common cause of turfgrass damage in football pitches and golf courses (Vandenbossche et al., 2011); and their presence on turfgrass results in a variety of symptoms that contribute to not only poor turf stands but also unsatisfactory turf quality even where damage may be considered to be fairly low.
There have been a number of plant-parasitic nematodes implicated in turfgrass damage (Yu et al., 1998). However, sting, lance, root-knot, spiral, stunt, ring, pin and dagger nematodes are widely known to be the most common and destructive groups (Crow, 2010). Generally, nematode problems are known to be more common in warmer seasons; conditions which allow efficient nematode reproduction, with faster generation turn over (Aryal et al., 2015). Nematodes in cool-season turfgrass do not normally cause direct damage unless other stress factors are involved, (for example, a synergistic effect of plant-parasitic nematodes and fungal pathogens) or when populations occur at extremely high densities (Thompson et al., 2000; Watschke et al., 2013). It should also be noted that turfs are normally better grown on sand-based soil, characteristics which ensure rapid drainage, reduce compaction, and improve playability. However, nematode activities are also reported to be more favored and enhanced in sandy soils (Crow, 2017). In Korea, a few studies have implicated nematodes in damage of turf on golf courses (Choo et al., 1998; Khan et al., 2008). Albeit the limited surveys on the occurrence of turf nematodes in Korea, spiral nematode, Helicotylenchus microlobus (79%) and ring nematode, Mesocriconema nebraskense (62%) have recently been reported to be the most prevalent species occurring on most of the golf courses in the country (Mwamula and Lee, 2021).
Zoysia spp. accounts for about 95.7% of the sod production in Korea. This is due to its high demand as a resource for establishing roadside slope covers and graveyard beautification (Bae et al., 2013). However, Kentucky blue-, Bent-, Bermuda- and Rye-grass are also produced, mainly for golf courses. In Korea, golf courses are mostly established using cool-season grasses, and Kentucky blue is given priority because of its cheap nature in terms of production and availability, and its ability to form dense lush and durable lawn that lives up to its reputation. Despite the high acceptability, Kentucky bluegrass requires a relatively high level of maintenance, and is also sensitive to parasitic nematodes. Turf infested with plant-parasitic nematodes can often persist with no visible damage but high population densities could lead to severe damage with symptoms like yellowing, wilting, browning, or thinning out. Other diseases and symptoms like brown patch (R. solani) are normally manifested on almost any cool season turf like Kentucky bluegrass and perennial ryegrasses while chances of damage are even higher on warm season grasses like Zoysiagrass; differing in only symptoms and severity of damage (Min et al. 2014).
Helicotylenchus spp. are one of the most ubiquitous plant-parasitic nematodes worldwide, normally associated with various horticultural, agronomic, and ornamental crops, including turfgrass (O’Bannon et al., 1989). On the other hand, Mesocriconema nebraskense is known to prefer grasses and woody perennials (Olson et al., 2017). The extensive host range of both nematode groups makes management generally difficult. Chemical control is currently the most reliable approach to reduce plant-parasitic nematode populations below threshold levels in most agricultural systems. Fenamiphos has been the predominantly used, most effective nematicide on golf courses, until the phasing out of its production in 2007 following a review on its safety profile by Environmental Protection Agencies (Fleming et al., 2008). Recent nematicides are considered to be much safer, with a relatively low risk to non-target organisms in the environment. This study therefore aimed at testing the efficacy of selected nematicides for the control of Helicotylenchus microlobus and Mesocriconema nebraskense on Kentucky bluegrass.
Materials and methods
Nematicides
Four different nematicides used in this study were fluopyram, imicyafos, fosthiazate, abamectin and ethoprophos. All these chemicals have a well-known nematicidal activity and have been tested and applied as nematode control chemical agents, especially in the past two decades (KCPA, 2016). Fluopyram is a non-fumigant fungicide widely used to control many fungal pathogens, including nematodes in various agricultural crops (Faske and Hurd, 2015). Ethoprophos is non-systemic, non-fumigant nematicide, which is also known to be effective against several other soil-borne insects, and is commonly used in Korea. On the other hand, imicyafos, fosthiazate and abamectin are non-fumigants; and are registered as nematicides against other plant-parasitic nematodes in Korea (KCPA, 2016). All the nematicides used in this study were obtained from local pesticide market.
Soil sampling
Soil samples were taken during the survey period of 2019 from Namhae South cape Country Club, located in 249 Jindong-ri, Changseon-myeon, Namhae-gun, Gyeongsangnam-do, South Korea. Areas with symptoms of nematode damage were given priority during sampling. Each sample (11 cm in diameter and 20 cm deep), was taken using a standard hole cutter, placed in plastic bags and stored at 4oC until analysis.
Nematode extraction, identification and counting
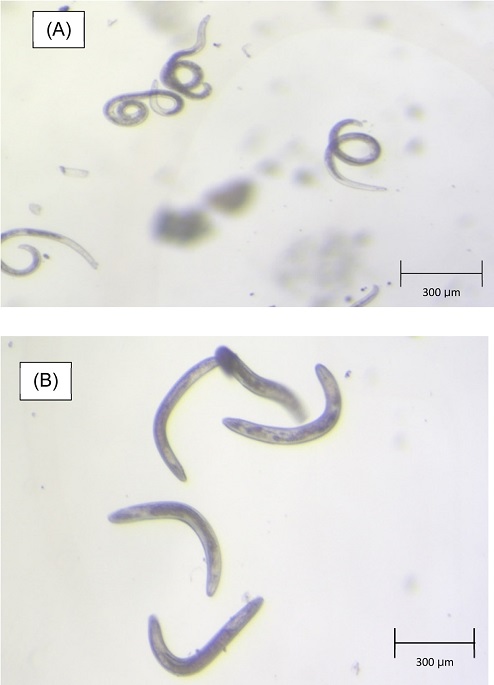
300-gram subsample was taken from previously collected field sample after thorough mixing. Nematodes were extracted from the soil by a combination of modified Cobb’s sieving and Baermann funnel methods (Jenkins, 1964). The collected nematode suspension was then examined under a stereomicroscope, Nikon SMZ 1000 (Nikon, Tokyo, Japan). Helicotylenchus microlobus and Mesocriconema nebraskense were identified and enumerated as promorphs (nematode forms which are easily recognizable at low magnification (less than ×100) under stereomicroscope) (Fig. 1). Species confirmation were done according to diagnostic criteria using morphological, morphometric and molecular tools as detailed by Mwamula et al. (2020a, 2020b).
Laboratory test
Nematicidal activity of four different nematicides against all the vermiform stages of the two species was tested. In laboratory conditions, a nematode suspension containing approximately 100 nematodes/ml of each species (free living nematodes inclusive, but not counted) was prepared and placed in each single well of a 12 multi-well culture plate. Each well was filled with 1 ml of the 4 different nematicides at different doses in 4 replicates. Details on the nematicides, their active ingredients and doses used at different concentrations are listed in Table 1. Four replicates of a control were also set up in the same way but with only water. The multi-well plates were then kept in growth chamber (Han Baek HB 303 DH-0, Korea) at 25ºC and the mortality of the two species was checked under a stereomicroscope (Nikon SMZ 1000, Nikon, Tokyo, Japan) 24, 48 and 72 hours after treatment.
Field test
Nematicidal efficacy of imicyafos, fosthiazate, fluopyram and ethoprophos against spiral and ring nematodes were tested in field conditions on Kentucky bluegrass under fairway at Namhae Southcape Country Club. In this experiment, initial population density of plant-parasitic nematodes (spiral and ring nematodes) and nematicidal efficacy (in terms of population reduction) in treated plots were compared with nematode populations recovered from untreated plots. Individual treatment plots comprising three replications; each sized (10 m × 1 m) were treated with imicyafos, fosthiazate, ethoprophos (60 g/10 m2) and fluopyram (100 g/10 m2). Soil samples were collected before treatment to count the initial nematode population, and 30 days after treatment to ascertain the effect of the chemicals on final nematode population. Samples were taken in the same way as described above.
Statistical analysis
Data from the laboratory tests were analyzed by logit/probit dose response/mortality regression; calculated using SAS statistical package version 9.4 (SAS Institute Inc., NC, USA). Treatment means were compared using Tukey's honestly significant difference (HSD) test at P ≤ 0.05. Corrected mortality was calculated using Microsoft Excel. Corrected mortality was also used to calculate lethal concentrations (LC) required to kill 50% (LC50) and 90% ( LC90) of the nematodes. For the field trials, nematode populations were determined at 30 days after treatment and the corrected population reduction was equally calculated.
Results
Acute toxicity of nematicides in laboratory conditions
Treatment of nematode populations with various concentrations of the nematicides showed variations in the acute toxicity of compounds depending on concentrations. Fluopyram showed a high control efficacy with LC50 and LC90 of as low as 0.0144 and 17.241 ppm, respectively after 72 hours of treatment (Table 2). Fosthiazate was the second most effective compound, also with LC50 and LC90 of as low as 1.30 and 51.44 ppm, respectively. Abamectin showed a moderate efficacy with LC50 at 19.6 ppm after 72 hours of treatment. However, the recorded LC90 required very high doses. On the other hand, just like abamectin, imicyafos showed similar results, with its LC50 recorded as 5.2 ppm, and a very high LC90 (14269 ppm) after 72 hours of treatment.
Correlation between corrected mortality and nematicide dose
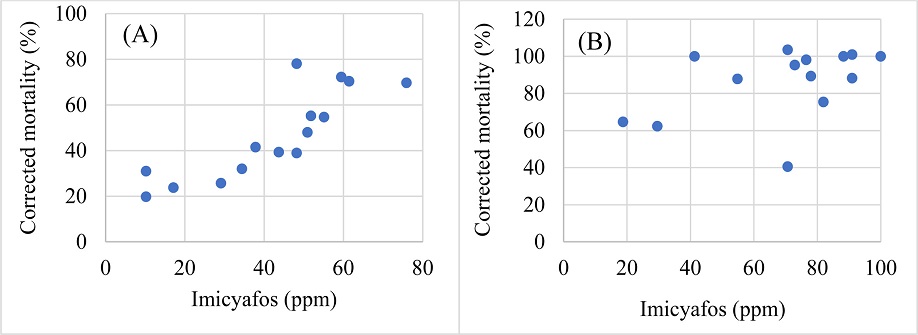
To visualize the nematicide-nematode mortality interactions, correlation between the tested doses of the chemicals and the corrected mortalities of Helicotylenchus microlobus and Mesocriconema nebraskense was tested. Commensurate with the moderate efficacy of abamectin, a moderate correlation between the tested doses of abamectin and corrected mortality of Helicotylenchus microlobus (R=0.793, df=13, P=0.0004) and Mesocriconema nebraskense (R=0.635, df=13, P=0.0109) was observed (Fig. 2A and 2B, respectively). Fluopyram doses / concentrations showed a moderate uphill relationship with the corrected mortality of Helicotylenchus microlobus (R=0.687, P=0.0046), while a weak uphill relation was observed when compared with corrected mortality of Mesocriconema nebraskense (R=0.249, P=0.0005) (Fig. 3). Additionally, fosthiazate concentrations showed a strong correlation with the corrected mortality of Helicotylenchus microlobus (Fig. 4A) (R=0.975) but the relation was not statistically significant (P=7.134). On the other hand, a comparison with corrected mortality of Mesocriconema nebraskense demonstrated a weak uphill linear relation (R=0.266) (Fig. 4B), which was also not statistically significant (P=0.3379). Likewise, a strong non-significant correlation between imicyafos dose / concentration and corrected mortality of Helicotylenchus microlobus was observed (Fig. 5A) (R=0.845, P=7.4502). However, a significant moderate correlation between imicyafos and Mesocriconema nebraskense was evident (R=0.707; P=0.0031) (Fig. 5B).
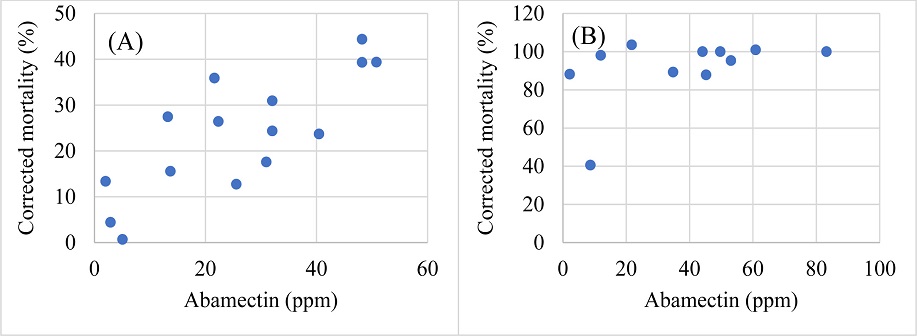
Linear regression of corrected mortality of Helicotylenchus microlobus (A) and Mesocriconema nebraskense (B). (P<0.05), when treated with abamectin.
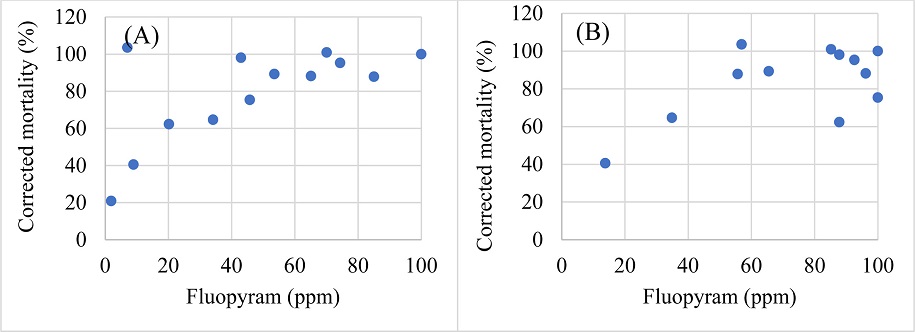
Linear regression of corrected mortality of Helicotylenchus microlobus (A) and Mesocriconema nebraskense (B), when treated with fluopyram.
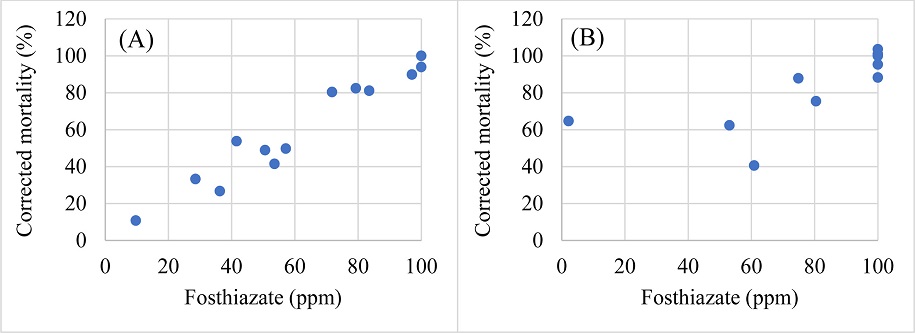
Linear regression of corrected mortality of Helicotylenchus microlobus (A) and Mesocriconema nebraskense (B), when treated with fosthiazate.
Effect of different nematicidal compounds in field conditions
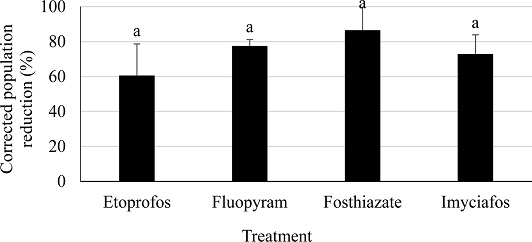
The corrected nematode population reduction rates of both the spiral and ring nematode varied differently depending on the nematicide (Fig. 6). Although the corrected population reduction among the treated nematicides were not statistically significant (df=3, 14, F=1.38, P<0.3162), fosthiazate showed the greatest population reduction (86%) among all the tested nematicides (Fig. 6) while ethoprophos showed the lowest nematode population reduction (60%).
Discussion
Abamectin, fluopyram, fosthiazate and imicyafos are comparatively newer chemicals that have been shown to suppress several nematode species and other agronomic pests (Kim et al., 2015; 2016). These chemicals have been used as insecticides and miticides for several years. However, regarding the control of nematodes using these compounds, most tests have been done on specific nematode groups like root-knot, root lesion, cyst and sting nematodes (Faske and Starr, 2006; Faske and Hurd, 2015; Crow et al., 2014; Kim et al., 2015; 2016). Among all the above listed nematicides tested in laboratory conditions, fluopyram showed the greatest efficacy and acute toxicity on the two studied nematode species. In our study, fluopyram was able to kill about 90% of the population with a dose of 77.31-17.24 ppm. Fluopyram is generally known to be effective against various plant-parasitic nematode groups on turfgrass and other agricultural crops (Crow et al., 2017). For example, fluopyram has been shown to reduce sting nematode populations on turfgrass and commercial strawberry (Petelewicz et al., 2020; Watson et al., 2020). Additionally, Faske and Hurd (2015) demonstrated that fluopyram was very effective at disrupting motility of Meloidogyne incognita and Rotylenchulus reniformis, hence reducing the ability of both nematode species to infect host roots.
Fosthiazate was depicted as the second most lethal compound on Helicotylenchus microlobus and Mesocriconema nebraskense. Fosthiazate, a granular non-fumigant nematicide, is widely accepted for its high efficacy, easy application and safer properties; mostly applied in the control of nematodes like cyst and root knot nematodes (Giblin-Davis et al., 1993; Lamberti et al., 2000). Whereas fosthiazate has been used in effective control of root-knot, cyst, and lesion nematodes in other cropping systems (Kim et al., 2016; Faske and Hurd, 2015), there have also been positive reports of its efficacy against plant-parasitic nematodes in turfgrass agroecosystems. For example, Giblin-Davis et al. (1993) demonstrated that fosthiazate was as effective as or even more effective than fenamiphos in reducing the soil populations of both the sting nematode (Belonolaimus longicaudatus) and root-knot nematodes (Meloidogyne spp.) on turfgrass.
In contrast, abamectin is known to be highly effective in suppressing different nematode populations at optimal concentrations (Crow et al., 2014). However, our results depicted that a higher concentration of abamectin were required to suppress / kill the tested nematode populations. The demonstrated outcome is comparable to the findings of Faske and Starr (2006) who reported that, much as the exposure of abamectin to Meloidogyne incognita resulted in irreversible paralysis and thus inhibited infection, the chemical showed less sensitive action against populations of Rotylenchulus reniformis with a much higher LD90. Similarly, our results demonstrated that imicyafos was also only effective at high doses, and efficacy depended on the exposure time (LC50= 5.2 ppm, and LC90 of as high as 14269.0 ppm after 72 hours of treatment). Imicyafos, a newly developed non-fumigant nematicide, is widely used in Korea and Japan to control cyst, root-knot and lesion nematodes (Wada et al., 2011; Kim et al., 2016; Huang et al., 2016). It should therefore be noted that, the efficacy of abamectin and imicyafos against plant-parasitic nematodes seems to vary depending on specific nematode species under investigation. Despite these findings, these compounds are still rated as excellent chemical options for nematode management, especially root-knot nematodes.
All the tested nematicides were able to reduce nematode populations by at least 60% in field conditions. Although, fluopyram showed high acute toxicity against the nematode populations in laboratory conditions, it ranked second (77.5% population reduction) to fosthiazate (82.5%). However, in the recent golf course experimental results published by Na et al. (2021), fluopyram was shown to effectively suppress mixed nematode populations (Paratrichodorus minor, Helicotylenchus microlobus, Paralongidorus koreanensis, Mesocriconema curvatum, and Tylenchorhynchus thermophilus) by about 81.8% population reduction after 60 days of treatment. Additionally, Faske and Hurd (2015) reported that the effect of fluopyram on motility of Meloidogyne incognita and Rotylenchulus reniformis were more pronounced compared to treatment of these species’ populations with either aldicarb or iprodione. Fluopyram is known to have an intermediate soil adsorption value, with an extremely long soil half-life of up to two years (Crow et al., 2017). The intermediate level of soil adsorption allows the chemical to move through the soil and its effects on the nematode population can be sustained for a long time in soil, thus providing longer nematode population suppression effect than other nematicides with short soil half-life.
On the other hand, fosthiazate is an acetylcholinesterase inhibitor that negatively affects nematode hatching and movement (Koyanagi et al., 1998; Woods et al., 1999). Whereas inhibition of acetylcholinesterase by oxime carbamates like aldicarb may be reversible, this process is thought to be irreversible with fosthiazate (Woods et al 1999). This could therefore explain the high efficacy of fosthiazate in the field compared to mortality tests in laboratory. In the field conditions, nematode reproduction and fitness depends on their ability to freely move in soil and find new hosts. The immobile condition caused by the mode of action of fosthiazate (inhibition of acetylcholinesterase) definitely reduces nematode fitness in addition to the eventual mortality, thus causing population suppressions. Imicyafos and ethoprophos showed comparatively lower mortality than fluopyram and fosthiazate. However, imicyafos has been shown to have a significant effect on other plant-parasitic nematodes like Pratylenchus penetrans (Wada et al., 2011) and Meloidogyne spp. (Kim et al., 2015). Therefore, its moderate action herein may be attributed to species specific attributes or variations in the required dosage for effective control of the nematode species under study, among other factors.
Regression coefficient results showed no negative relation between the tested nematicides and nematode mortality. The results therefore demonstrated that there is strong correlation between nematicide treatment and corrected mortality of especially Helicotylenchus microlobus. The weak uphill relationships observed between nematicide treatment and mortality of Mesocriconema nebraskense may be explained in terms of differences in nematicide properties, influence of soil properties, nematode-chemical tolerance abilities and possible variations in the required dosage for effective control of the nematode species (Crow et al., 2017). Control of nematodes in turfgrass systems is influenced by many factors, including the differences in migration patterns of the different nematode species. For example, ring nematodes are known to occupy lower soil layers during cold seasons including spring, and they move higher up in the soil profile during summer (Crow et al., 2017). Nematicides like abamectin, imicyafos and even fluopyram have major limitations: Abamectin has a very low water solubility, yet with an extremely high soil adsorption value; these properties do not allow it to move well within the soil (Crow et al., 2017). Wada et al., (2011) also reported that unlike soil fumiganttype nematicides which transcend through even the deeper soil layers, the effectiveness of imicyafos on the soil nematode communities is highly limited to the surface soil layer. Fluopyram is also a contact nematicide, even though it is known to be taken up by roots to control fungal foliar diseases (Crow et al., 2017). Therefore, these compounds especially abamectin and imicyafos, when sprayed onto the turfgrass surfaces and irrigated, very little amounts of the chemicals make it through the turf thatch, and therefore may only show effectiveness on nematode populations near the soil surface. The current field tests were performed during the spring season, and this could explain the differences in the sensitivity of the ring nematodes to the tested nematicides. Thus, more controlled pot tests under controlled environment conditions in green house involving tests of the chemical on pure cultured population of Mesocriconema nebraskense may provide a more accurate interaction between the nematode and the tested nematicides. However, this study demonstrated that these chemical compounds are good alternatives for the nematode management in turfgrass.
Acknowledgments
This research was supported by the Gyeongsangbuk-do Office of Education's ‘15th Future Scientists Training Program’. We would like to thank Hee-woo Kim at South Cape Golf and Resort in Namhae for helping us in performing field tests on the golf course and Ho-wook Lee for helping us during the experiment.
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
References
-
Amiri A, Mulvaney KA, Pandit LK, Angelis DR. 2017, First report of resistance to fluxapyroxad and fluopyram in Botrytis cinerea from commercial apple orchards in Washington State. Plant Dis. 101(3):508-508.
[https://doi.org/10.1094/PDIS-09-16-1384-PDN]

- Aryal S, Crow WT, McSorley R, Giblin-Davis RM. Kenworthy KE, 2014. Integrated pest management of sting nematode (Belonolaimus longicaudatus) on bermudagrass. Phytopathol. 104:10-10.
-
Bae EJ, Lee KS, Kim DS, Han EH, Lee SM, et al., 2013. Sod production and current status of cultivation management in Korea. Weed Turf. Sci. 2(1):95-99.
[https://doi.org/10.5660/WTS.2013.2.1.095]

- Becker JO, Ploeg A, 2013. Efficacy of nematicides for root-knot nematode management in tomato, 2012. Plant Dis. Manag. Rep. 7:N009.
- Brandenburg RL, Freeman CP, 2012. Handbook of turfgrass insects. 2nd ed. Entomological Society of America. Lanham, USA. 63pp.
-
Choo HY, Lee DW, 2017. Research review on turfgrass insect pests in Korea. Weed Turf. Sci. 6(2):77-85. In Korean)
[https://doi.org/10.5660/WTS.2017.6.2.77]

- Choo HY, Lee DW, Kim HH, Park JW, Sung YT, Chung YK, 1998. A newly recorded turfgrass pest, root-knot nematode, Meloidogyne incognita. Korean golf courses. Kor. Turfgrass Sci. 12:107-112.
- Crow B, 2017. Nematodes-How do I know if I have a problem? Green Section Record 55(9):1-6. (http://www.greencastonline.com/promo/nematode-knowledge/images/usga-nematodes-crow-5-2017.pdf, ).
-
Crow WT, 2005. Plant-parasitic nematodes on golf course turf. Outlooks on Pest Manage. 16(1):10-15.
[https://doi.org/10.1564/16feb04]

-
Crow WT, 2007. Understanding and managing plant-parasitic nematodes on turfgrasses. In Handbook of Turfgrass Management and Physiology, eds. by CRC Press, pp. 362-382. USA.
[https://doi.org/10.1201/9781420006483.ch22]

- Crow WT, 2010. Nematode management for golf courses in Florida. EDIS, 2010:2. (http://edis.ifas.ufl.edu/in124, ).
- Crow WT, Becker JO, Baird JH, 2017. New golf course nematicides. Golf Course Management, 7:66-71.
- Crow WT, Dant L, 2014. Treatment zone of abamectin in golf course greens. J. Nematol. 46:149-149.
- Faske TR, Hurd K, 2015. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to fluopyram. J. Nematol. 47(4):316-321.
- Faske TR, Starr JL, 2006. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to abamectin. J. Nematol. 38(2):240-244.
- Fleming CC, Craig D, Hainon-McDowell M, Entwistle K, 2008. Plant parasitic nematodes-a new turf war?. Biologist-Institute of Biology, 55(2):76-82.
- Giblin-Davis RM, Cisar JL, Bilz FG, 1993. Evaluation of fosthiazate for the suppression of phytoparasitic nematodes in turfgrass. Nematropica. 23(2):167-175.
-
Huang WK, Wu QS, Peng H, Kong LA, Liu SM, et al., 2016. Mutations in acetylcholinesterase2 (ace2) increase the insensitivity of acetylcholinesterase to fosthiazate in the root-knot nematode Meloidogyne incognita. Sci. Rep. 6:38102.
[https://doi.org/10.1038/srep38102]

- Jenkins WR, 1964. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 48(9):692.
-
Kang B, Kabir FM, Bae EJ, Lee GS, Jeon B, et al., 2016. Damage report on a newly recorded coleopteran pest, Aphanisticus congener (Coleoptera: Buprestidae) from turfgrass in Korea. Weed Turf. Sci. 5(4):274-279.
[https://doi.org/10.5660/WTS.2016.5.4.274]

-
Khan Z, Kim JH, Son SH, Kim SG, Kim YH, 2008. Occurrence of Stunt Nematode, Tylenchorhynchus claytoni, on Turfgrass in Korea. Plant Pathol. J. 24(4):474-477.
[https://doi.org/10.5423/PPJ.2008.24.4.474]

-
Kim HH, Jung YH, Kim DH, Ha TK, Yoon JB, et al., 2015. Control effects of imicyafos GR against two species of the root-knot nematodes (Meloidogyne incognita and Meloidogyne hapla). Korean J. Pestic. Sci. 19(2):101-105.
[https://doi.org/10.7585/kjps.2015.19.2.101]

- Kim HK, 1991. Turfgrass science. pp. 545. Sunjinmunhwasa. Seoul. Korea. (In Korean).
-
Kim J, Mwamula AO, Kabir MF, Shin JH, Choi YH, et al., 2016. Efficacy of different nematicidal compounds on hatching and mortality of Heterodera schachtii infective juveniles. Korean J. Pestic. Sci. 20(4):293-299.
[https://doi.org/10.7585/kjps.2016.20.4.293]

- Korea Crop Protection Association (KCPA), 2016. 2016 pesticide manual. Korea Crop Protection Association. Seoul, Korea. (In Korean).
-
Koyanagi T, Imai O, Yoshida K, 1998. Development of a new nematicide, fosthiazate. J. Pestic. Sci. 23(2):174-183.
[https://doi.org/10.1584/jpestics.23.174]

-
Lamberti F, D'Addabbo T, Sasanelli N, Carella A, Greco P, 2000. Chemical control of root-knot nematodes. Acta Hort. 532:183-188.
[https://doi.org/10.17660/ActaHortic.2000.532.23]

-
Lee CM, Kwon OG, Lee KS, Lee SM, Choi S, et al., 2014. Insect pests in turf sod production areas in Korea. Weed Turf. Sci. 3(2):114-120.
[https://doi.org/10.5660/WTS.2014.3.2.114]

-
Min GY, Lee JH, Kwak YS, 2014. A detail investigation of major diseases occurrence and pathogen population on turfgrass cultivation in nationwide. Weed Turf. Sci. 3(2):121-129.
[https://doi.org/10.5660/WTS.2014.3.2.121]

- Moore SR, Lawrence KS, 2010. Evaluation of iprodione for control of root-knot nematodes on tomato in south Alabama, 2009. Plant Dis. Manag. Rep. 4:N012.
-
Mwamula AO, Kabir MF, Lee G, Choi IH, Kim YH, et al., 2020a. Morphological characterisation and molecular phylogeny of several species of Criconematina (Nematoda: Tylenchida) associated with turfgrass in Korea, as inferred from ribosomal and mitochondrial DNA. Nematology, 22:939-956.
[https://doi.org/10.1163/15685411-bja10003]

-
Mwamula AO, Na H, Kim YH, Kim YH, Han G, et al., 2020b. Characterization of a new spiral nematode, Helicotylenchus asiaticus n. sp., and three known species from Korea; with comments on the validity of Helicotylenchus microlobus Perry in Perry, Darling & Thorne, 1959. Eur. J. Plant Pathol. 157:565-581.
[https://doi.org/10.1007/s10658-020-02022-9]

-
Mwamula AO, Lee DW, 2021. Occurrence of Plant-parasitic Nematodes of Turfgrass in Korea. Plant Pathol. J. In press.
[https://doi.org/10.5423/PPJ.OA.04.2021.0059]

- Na HB, Mwamula AO, Bae EJ, Lee DW., 2021. Efficacy of some nematicidal agents against turfgrass parasitic nematodes. Weed Turf. Sci. 10(1):65-78.
- O’Bannon JH, Inserra RN, 1989. Helicotylenchus species as crop damaging parasitic nematodes. Nematology Circular. 165:1-3.
-
Olson M, Harris T, Higgins R, Mullin P, Powers K, et al., 2017. Species delimitation and description of Mesocriconema nebraskense n. sp. (Nematoda: Criconematidae), a morphologically cryptic, parthenogenetic species from North American grasslands. J. Nematol. 49(1):42-66.
[https://doi.org/10.21307/jofnem-2017-045]

-
Petelewicz P, Orliński PM, Schiavon M, Mundo-Ocampo M, Becker JO, Baird JH, 2020. Fluopyram Controls Shoot-galling Caused by Pacific Shoot-gall Nematode and Improves Turf Quality in Annual Bluegrass Putting Greens. Hort Technology, 30(6):709-718.
[https://doi.org/10.21273/HORTTECH04680-20]

-
Thompson JP, Greco N, Eastwood R, Sharma SB, Scurrah M, 2000. Integrated control of nematodes of cool season food legumes. pp. 491-506. In Linking Research and Marketing Opportunities for Pulses in the 21st Century. (Ed. Knight R.), Springer, Dordrecht, Germany.
[https://doi.org/10.1007/978-94-011-4385-1_45]

-
Vandenbossche B, Viaene N, Sutter N, Maes M, Karssen G, et al., 2011. Diversity and incidence of plant-parasitic nematodes in Belgian turf grass. Nematology. 13(2):245-256.
[https://doi.org/10.1163/138855410X517084]

- Wada S, Toyota K, Takada A, 2011. Effects of the nematicide imicyafos on soil nematode community structure and damage to radish caused by Pratylenchus penetrans. J. Nematol. 43(1):1-6.
-
Watschke TL, Dernoeden PH, Shetlar DJ, 2013. Managing turfgrass pests. 2nd ed. CRC Press, Florida, USA. 193pp.
[https://doi.org/10.1201/b14741]

-
Watson TT, Noling JW, Desaeger JA, 2020. Fluopyram as a rescue nematicide for managing sting nematode (Belonolaimus longicaudatus) on commercial strawberry in Florida. Crop Prot. 132:105108.
[https://doi.org/10.1016/j.cropro.2020.105108]

-
Woods SR, Haydock PPJ, Edmunds C, 1999. Mode of action of fosthiazate used for the control of the potato cyst nematode Globodera pallida. Ann. Appl. Biol. 135(1):409-415.
[https://doi.org/10.1111/j.1744-7348.1999.tb00868.x]

-
Yu Q, Potter JW, Gilby G, 1998. Plant-parasitic nematodes associated with turfgrass in golf courses in southern Ontario. Canadian J. Plant Pathol. 20:304-307.
[https://doi.org/10.1080/07060669809500397]

Md. Faisal Kabir, and Abraham Okki Mwamula, Department of Ecological Science, Kyungpook National University, PhD.
Heebeen Na, In Ho Choi, and Yoon Suk Cha, Department of Ecological Science, Kyungpook National University, Master student
Young Gyun Kim, Geun Wook Lee, and Gyeongman Lee, Gyeong-gu High School, Student.
Kyoung Ae Kim, Gyeong-gu High School, Teacher.
DongWoon Lee, Department of Ecological Science, Kyungpook National University, Professor, ORCID http://orcid.org/0000-0001-9751-5390.
Research design; Lee DW, Kim KA, Investigation; Kabir MF, Na H, Choi IH, Cha YS, Mwamula AO, Kim YG, Lee GW, Lee G, Formal analysis; Na H, Kabir MF, Writing-original draft preparation; Lee DW, Kabir MF, Writing-review and editing; Lee DW, Mwamula AO