Effects of Temperature and Overwintering on Insecticide Toxicity to Small Brown Planthopper
 ; Sung-Wook Jeon
; Sung-Wook Jeon ; Sang-Ku Lee1 ; Si Woo Lee2 ; Deok Ho Kwon2 ; Young Ho Koh3
; Sang-Ku Lee1 ; Si Woo Lee2 ; Deok Ho Kwon2 ; Young Ho Koh3 ; Si Hyeock Lee4
; Si Hyeock Lee4
Abstract
Temperature is one of the most important factors that affect insecticide toxicity and daily and seasonal fluctuation of insect occurrence in crop field. However, there is little information on the relationship between the temperature and insecticide toxicity of the small brown planthopper (SBPH). In this study, the influence of temperature on the toxicity of insecticide was examined. The toxicities of fipronil and three neonicotinoids, dinotefuran, imidacloprid, and thiamethoxam were found to be positive temperature coefficient and that of etofenprox was negative one. Carbamates (carbosulfan and fenobucarb) were showed no relations with temperature increase. As for the resistant SBPH strains, the toxicity ratio (RT) between 20 and 30oC of imidacloprid-resistant strain exhibited much higher (204.4 - fold) than that (1.0 - fold) of etofenprox-resistant one. The mean mortality of after-overwintering populations showed also significantly higher than that of before-overwintering populations. This study provides a clue to the effect of temperature rise on insecticide efficacy and gives the theoretical basis for the effective use of chemical insecticides and resistance management to control SBPH suffering the overwintering.
Keywords:
Insecticide efficacy, Overwinter, Resistance, Small brown planthopperIntroduction
Temperature plays a crucial role in influencing the effectiveness of insecticides. It influences insect survival and population growth through alteration of fertility and fecundity rates, including survival and adult life-span (Dreyer and Baumagartners, 2000; Zhen et al., 2008). Relationships of temperature and toxicity within many species have been demonstrated in various studies (Give examples of publications by citing here). The continuous increase in temperature caused by climate change steadily influences the morphology, physiology and ecology of insect pests including insecticide resistance of indigenous insects by improving the adaptability of migratory insects to invade the once colder unfavorable regions. This has contributed to increased crop losses by insect pests in especially the temperate regions (Deutsch et al., 2018).
The Small brown planthopper (SBPH) Laodelphax striatellus Fallén (Hemiptera: Delphacidae) is one of the most serious pests in rice -fields in Asia. It destroys crops by sap-sucking behavior using its piercing-sucking mouthpart and also transmits various plant viruses, such as rice stripe virus (RSV), rice black-streaked dwarf virus (RBSDV), among others (Choi et al., 2018; Otuka, 2013). In Korea, SBPHs are divided into two groups; the overwintered indigenous SBPH populations, and migrated SBPH populations which migrate from China through seasonal winds from May to July (Hyun et al., 1977; Jeong et al., 2018).
The control of SBPH mostly rely on chemical insecticides including organophosphates, carbamates, pyrethroids, and neonicotinoids (Lee et al., 2005; Park et al., 2009). However, the insecticide resistance recorded in SBPH continues to reduce the reliability of many of the available insecticides due to negative changes in chemical toxicity. The change of toxicity due to temperature can be positive or negative, and depends on the differences in the mode of action, exposure routes of insecticide and target insects (Gordon, 2005). Pyrethroid and neonicotinoid insecticide classes have usually been reported to have negative and positive temperature coefficient, respectively in Nilaparvata lugens (Mao et al., 2019). In addition, some studies have reported that resistant strains show poor winter survival, maladaptive behavior and reduced reproductive fitness (Foster et al., 2000). They differ from the susceptible strains in some biological advantages such as high adaptation ability to insecticide and survival in insecticide exposure, and finally replace the susceptible strains. It is therefore important to critically consider the effects of temperature on insecticide efficacy as a way of ensuring efficiency in the use of insecticides and subsequent reduction of the foreseeable impacts of insect resistance. However, the effects of temperature on insecticidal toxicity to SBPH has not been intensively studied.
Therefore, in this study, the effect of temperature on the effectiveness of commercial insecticides was assessed after exposing SBPHs to different temperatures, and the change of insecticide toxicity to local SBPH populations after overwintering was also checked. From these results, some important suggestions can be proposed for the future insect control methods and insecticide study.
Materials and Methods
Insecticides and susceptible SBPH strain
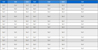
Six insecticides (carbosulfan, 20% SC; fenobucarb, 50% EC; etofenprox, 20% EC; dinotefuran, 10% WP; imidacloprid, 10% WP; thiamethoxam, 10% WG) registered for SBPH control in Korea were purchased commercially, and fipronil (5% EC) was experimentally provided by Sungbo Chemical Co. Ltd (Korea) instead of the registered 0.5% FG formulation (Table 1). The susceptible SBPH (Sus-SBPH) strain maintained without exposure to any insecticide for ten years in an insectarium (Division of Crop protection, National Institute of Agricultural Sciences) in acryl cage (30 × 22 × 30 cm3) by serving seedlings of Chucheong rice variety conditioned at 25 ± 2oC, 50-60% relative humidity, and a 16:8 (L:D) photoperiod was used (Jeong et al., 2017). Egg-laid rice seedling were divided in five groups and hatched in different temperature condition (15, 20, 25, 30 and 35oC). The 3rd nymphs were introduced into bioassay.
Median lethal concentration in different temperatures
Bioassay was conducted by rice-seedling dipping (Jeong et al., 2017). Rice (Chucheong) was seeded on the seedling pot, and young seedling stem was used for assay after 3-4 weeks. The seedlings were picked up from the pot, washed, and the seedling stems (7 cm long) were cut without damaging the whole root after being dried in the shade. Five stems were packed to one batch and soaked in designated concentration of insecticides for 30 seconds and dried in the shade. The roots were then rolled with sanitary cotton and put into the glass test tube (φ27 × h200 mm2) and 15 nymphs of 3 days old were introduced into the glass tube and dead insects were counted at 72 h after treatment. Every treatment was performed in triplicate. For calculating median lethal concentration (LC50), statistical program of SPSS 13.0 (IBM Analytics, Armonk, NY) was used.
Resistant SBPH strains and their resistance ratio with temperature
Two insecticide-resistant SBPH strains were previously established in our published paper (Jeong et al., 2021). Briefly, SBPHs collected from the rice paddies in 2018 were divided into two groups and then treated with either imidacloprid (10% WP) or etofenprox (20% EC) with LC30 concentrations (5 ppm and 25 ppm, respectively) and total 7-9 selections were conducted. The resistance ratio (RR) of the imidacloprid-resistant SBPH strain (ImR-SBPH) and the etofenprox-resistant SBPH one (EtR-SBPH) was 88.9 and 48.1, respectively (Table 2).
The 3rd instar nymphs of one susceptible (Sus-SBPH) and two resistance strains (ImR-SBPH and EtR-SBPH) were introduced to bioassay with rice-stem dipping method previously described. The exposure temperature was only different with 20 and 30oC after 72 h insecticide treatment. These two temperatures were selected in consideration of the range (18-32oC) of average monthly temperature from April to September. Two insecticides, imidacloprid WP and etofenprox EC were treated to the imidacloprid strains and etofenprox strains, respectively. For calculating median lethal concentration (LC50), statistical probit analysis of SPSS 13.0 (IBM Analytics, Armonk, NY) was used and the resistance ratios of the populations were compared.
Single concentration diagnosis and overwintering effect
The 14 populations of SBPH were collected in the early winter of 2018 (before-overwintering population) and the spring of 2019 (after-overwintering population) at seven local areas, Chungju (CB_CJ), Sangju (GB_SJ), Yeongcheon (GB_YC), Taean (CN_TA), Buan (GB_BA), Shinan (JN_SA) and Jindo (JN_JD). The 3rd nymphs of populations collected were reared two generations in laboratory.
Five insecticides (carbosulfan, 20% SC; etofenprox, 20% EC; dinotefuran, 10% WP; imidacloprid, 10% WP; fipronil, 5% EC) were used to study insecticide responses (Table 1) and each diagnostic concentration (DC) (40 ppm, 10 ppm, 50 ppm, 300 ppm and 3 ppm, respectively) was deduced from the two times concentration of LC90 value to susceptible SBPH. Bioassay was conducted by the rice-seedling dipping method (Jeong et al., 2017) with the same materials and procedures. After 72 h of insecticide treatment, dead insects were counted for the calculation of mortality based on Abbott’s formula (Abbott, 1925). The differences between mortality of the before- and after-overwintering populations in each area were compared and analyzed by statistical t-test using SPSS 13.0 (IBM Analytics, Armonk, NY).
Results and Discussion
Temperature related insecticide responses
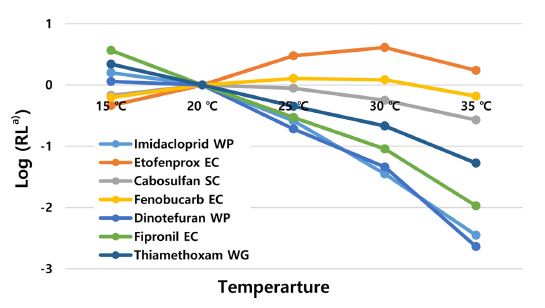
To explore the influence of temperature on the toxicity of insecticide, the toxicities of five insecticides to SBPH under different temperature conditions were determined (Table 3). Phenylpyrazole and neonicotinoids demonstrated positive temperature coefficients. Based on LC50 values, the toxicity of fipronil increased 3.7, 12.5, 40.5 and 345.2 folds at each sequential temperature (20, 25, 30 and 35oC) when compared with the toxicity at 15oC. Similarly, the toxicities of three neonicotinoids showed a significant increase (1.1, 5.9, 24.8 and 494.3 folds for dinotefuran, 1.6, 6.1, 44.8 and 446.6 folds for imidacloprid, 2.2, 4.9, 10.2 and 41.3 folds for thiamethoxam) when compared with their toxicity at 15oC. These results were similar to the results shown in other studies involving other pest insects (Boina et al., 2009; Mao et al., 2019). Therefore, the higher the temperature, the better the control efficiency. In contrast, the toxicity of pyrethroid, etofenprox showed negative temperature coefficient and its toxicity of decreased by 2.2, 6.5, 8.9 and 3.8 folds at each sequential temperature when compared with the toxicity at 15oC. This is in agreement with published findings from studies involving other pyrethroid insecticides (Mao et al., 2019) and pyrethroid insecticides will perform better efficacy at low temperatures in the field. Carbamates (carbosulfan and fenobucarb) showed no relations with temperature increase.
When compared with the toxicity at the temperature of 20oC (about average temperature ranged from 16 to 26oC in May to June in Korea), fipronil and neonicotinoid insecticides would be more recommended for SBPH management than pyrethroid insecticides (Fig. 1). Many researchers have suggested that the toxicity change of insecticides on the different temperature is related with the penetration and biotransformation of insecticides (Harwood et al., 2009), nerve impulse movement (Song and Narahasi, 1996), feeding consumption rate (Mao et al., 2019), detoxification and metabolic mechanism of insects (Cui et al., 2012; Shao et al., 2013). However, information on the toxicity dynamics of insecticides with temperature change is scarce. The assessment of temperature effects on the toxicity of different insecticides against a target insect is important to establish the chemical control strategies in given environmental conditions.
Effect of temperature on insecticide toxicity to the resistant SBPH strains
To determine the influence of temperature on the toxicity of insecticide to the resistance SBPH strains, the toxicities of two strains, imidacloprid-resistant and etofenprox-resistant SBPH were assessed under different temperatures of 20 and 30oC (Table 4). The toxicity ratio (TR) between the two temperatures of imidacloprid-resistant strain was higher (204.4) than that (1.0) of etofenprox-resistant strains. The resistance ratio (RR) of two strains decreased from 94.4 to 5.3 times in imidacloprid-resistant strain and from 38.3 to 16.9 times in etofenprox-resistant strain as temperature rises, respectively. The toxicity of the imidacloprid-resistant strain increased highly with temperature rise, which is similar to that of the susceptible strain, but the etofenprox-resistant strain showed no change in toxicity with temperature increase. The resistance ratio (RR) of the two resistant strains decreased with temperature increase, which indicates the toxic activity of the resistant strain is more sensitive to temperature change than that of the susceptible strain. Previously we reported that there was no strong correlation between the activities of the detoxifying enzymes (total esterase, acetylcholinesterase and glutathione S-transferase) and temperature changes in the various SBPHs. In contrast, the amounts of ROS, which were more abundant in the two resistant SBPH strains than in the Sus-SBPH strain, showed a strong correlation with temperature changes in all the strains, suggesting that ROS can play an important role in the temperature-related resistance change of SBPH (Jeong et al., 2021). This finding that resistance intensity can change with temperature has implications for the dynamics of resistance management. More researches on the biochemical, physiological, and genetic changes in a wide range of insects are needed to understand the insecticide resistance associated with temperature.
Effect of overwintering on insecticide toxicity to the local SBPH populations
To compare the changes of insecticide toxicity with overwintering, the mortality effects of five insecticides on 14 populations in seven location collected before and after overwintering seasons were evaluated (Table 5). The etofenprox showed the highest mortality in Taean population (89.8 %) and low in other populations (29.6 - 60.5%) in the before-overwintering season. After overwintering, its mortality increased in Chungju, Sangju, Yeongcheon; maintained in Buan, and decreased in Taean, Shinan and Jindo. The mortality rate of carbosulfan showed a more increasing change in all location from a range of 10.0 - 67.9 % to 50.0 -79.2%. The mortality rates of dinotefuran and imidacloprid have large increased in all locations after overwintering. Dinotefuran and imidacloprid showed low mortality range of 25.6 - 66.7% and 3.5 - 60.5% in the before-overwintering season and high mortality range of 61.2 - 95.9 % and 60.4 - 94.9 % in the after-overwintering season, respectively. The mortality of fipronil increased in Chungju, Sangju, Yeongcheon, maintained in Taean, and decreased in Buan, Shinan and Jindo. Although the efficacy of five pesticides has not increased on all the local populations, the mean mortality (66.3%) of after-overwintering populations were significantly higher than that (35.1%) of the before ones (Fig. 2).
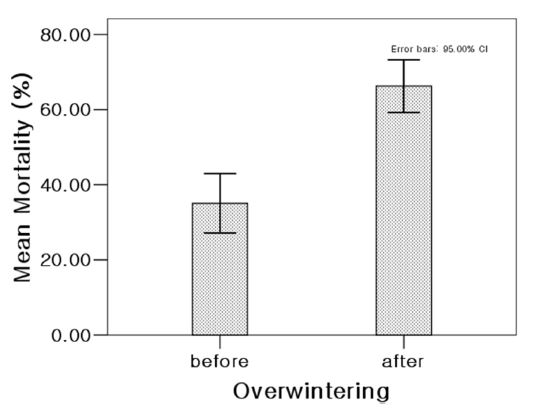
Mean mortality of seven local SBPH populations against five insecticides before- and after-overwintering.
Overwintering is the crucial factor for the survival of insect in the subtropical and temperate climate zones with stressful environmental condition, e.g., unavailable food resources, low temperatures or water deficits (Williams et al., 2015). The low temperature during winter can affect the mortality of insects due to severe tissue damages or accumulated chill injuries (Turnock and Fields, 2005). The overwintering mortality rates are influenced by many factors besides the low winter temperature.
Fitness costs associated with insecticide resistance are accompanied by high energetic cost or significant disadvantage that diminishes the insect’s fitness compared with its susceptible counterparts in the population. This fitness costs are known to affect differences with resistant and susceptible strains in some properties, such as developmental time, fecundity and fertility, sensitivity to alarm pheromone, and overwintering success (Talebi et al., 2015). In this study, the efficacy of five insecticides on SBPH showed the tendency to rise after overwintering, which suggested that the resistant SBPH would be more susceptible to overwintering (Fig. 2). Apparently, when fitness costs are high at low temperatures, relaxation of selection pressure is likely to favor a population’s reversion to susceptibility in regions of a relatively cold winter. Understanding the relationship of fitness cost and insecticidal resistance could be useful to design effective insecticide resistance management strategies. Overwintering would be helpful to lower the severity of resistance problem in domestic population, most of all, monitoring the migratory SBPH populations which move from China to Korea and mix with domestic populations every year would be especially emphasized.
Conclusion
Temperature changes due to the global warming can affect the population growth, overwintering, migration pattern and insecticide resistance accumulation of pests. It can influence the insecticide efficacy to field pest populations and lead to fail in controlling on time. Therefore, it is important to adjust the insect management strategies to the changes of annual occurrence, winter survival, migration and distribution of SBPH. The evaluation of resistance status and selection of effective insecticides with temperature rise are also inevitable in insect pest management.
Acknowledgments
This study was carried out with the support of “Research Program for Agricultural Science & Technology Development (Project No. PJ0108212019)”, National Institute of Agricultural Science, Rural Development Administration, Republic of Korea.
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
References
-
Abbott WS, 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18(2):265-267.
[https://doi.org/10.1093/jee/18.2.265a]

-
Boina DR, Onagbola EO, Salyani M, Stelinski LL, 2009. Influence of posttreatment temperature on the toxicity of insecticides against Diaphorina citri (Hemipter: Psyllidae). J. Econ. Entomol. 102(2):685-691.
[https://doi.org/10.1603/029.102.0229]

-
Choi JE, Kwak HR, Kim MK, Jeong TW, Seo JK, et al., 2018. Investigation of viruliferous insect rate of planthoppers captured by smart sky net trap (SSNT) in Korea during 2015-2017. Res. Plant Dis. 24(3):202-212. (In Korean)
[https://doi.org/10.5423/RPD.2018.24.3.202]

-
Cui L, Sun L, Yang D, Yan X, Yuan H, 2012. Effects of cycloxaprid, a novel cis-nitromethylene neonicotinoid insecticide, on the feeding behaviour of Sitobion avenae. Pest. Mang. Sci. 68(11):1484-1491.
[https://doi.org/10.1002/ps.3333]

-
Deutsch CA, Tewksbury JJ, Tigchelaar M, Battisti DS, Merrill SC, et al., 2018. Increase in crop losses to insect pests in a warming climate. Science. 361(6405):916-919.
[https://doi.org/10.1126/science.aat3466]

-
Dreyer H, Baumagartner J, 1996. Temperature influence on cohort parameters and demographic characteristics of the two cowpea coreids Clavigralla tomen tosicollis and C. shadabi. Entomol. Exp. Appl. 78(2):201-213.
[https://doi.org/10.1111/j.1570-7458.1996.tb00783.x]

-
Foster SP, Denholm I, Devonshire AL, 2000. The ups and downs of insecticide resistance in peach-potato aphid (Myzus persicae) in the UK. Crop Protection. 19(8-10):873-879.
[https://doi.org/10.1016/S0261-2194(00)00115-0]

- Gordon CJ, 2005. Temperature and toxicology: an interative, comparative and environmental approach. CRC Press, Boca Raton, Florida, USA. Pp. 233-264.
-
Harwood AD, You J, Lydy MJ, 2009. Temperature as a toxicity indentification evaluation tool for pyrethroides: toxicokinetic confirmation. Environ. Toxicol. Chem. 28(5):1051-1058.
[https://doi.org/10.1897/08-291.1]

- Hyun JS, Woo KS, Ryoo MI. 1977. Studies on the seasonal increase of the population of the small brown planthopper, Laodelphax striatellus (Fallen). Korean J. Appl. Entomol. 16(1):13-19.
-
Jeong IH, Jeon SW, Lee SK, Park B, Park SK, et al., 2017. Insecticide cross-resistance and developmental characteristics on the two rice varieties, ‘Chinnong’ and ‘Chuchung’, of the imidacloprid-resistanct brown planthopper. Korean J. Pestic. Sci. 21(4):381-388. (In Korean)
[https://doi.org/10.7585/kjps.2017.21.4.381]

-
Jeong IH, Jeon SW, Park B, Park SK, Lee SG, et al., 2018. Comparison of insecticide susceptibility between overwinter and migratory populations of Laodelphax striatellus collected in 2017. Korean J. Pestic. Sci. 22(4):261-268. (In Korean)
[https://doi.org/10.7585/kjps.2018.22.4.261]

-
Jeong IH, Kim AY, Nguyen P, Kwon DH, Koh YH, 2021. Temperature-indefendent increase in the detoxifying enzyme activity of insecticide-resistant small brown planthppers and Drosophila. J. Asia-Pacific Entomol. 24(1):70-76.
[https://doi.org/10.1016/j.aspen.2020.11.009]

- Lee SW, Choi BR, Park HM, Yoo JK, 2005. Monitoring on insecticide resistance of major insect pests in paddy field. Korean J. Pestic. Sci 9(4):365-373. (In Korean)
-
Mao K, Jin R, Li W, Ren Z, Qin X., et al., 2019. The influence of temperature on the toxicity of insecticides to Nilaparvata lugens Stal). Pestic. Biochem. Physiol. 156:80-86.
[https://doi.org/10.1016/j.pestbp.2019.02.009]

-
Otuka, A., 2013. Migration of rice planthoppers and their vectored re-emerging and novel rice viruses in East Asia. Front. Microbiol. 4:309-319.
[https://doi.org/10.3389/fmicb.2013.00309]

- Park JW, Jin TS, Choi HS, Lee SH, Shin DB, et al., 2009. Incidence of rice stripe virus during 2002 to 2004 in Korea and chemical control of small brown planthopper. Korean J. Pestic. Sci 13(4):309-314. (In Korean)
-
Shao X, Sweenson TL, Casida JE, 2013. Cycloxaprid insecticide: nicotinic acetylcholine receptor binding site and metabolism. J. Agric. Food Chem. 61(33):7883-7888.
[https://doi.org/10.1021/jf4030695]

- Song J, Narhashi T, 1996. Modulation of sodium channels of rat cerebella Purkinje neurons by the pyrethroid tetramethrin. J. Pharmacol. Exp. Ther. 277(1):445-453.
-
Turnock WJ, Fields PG, 2005. Winter climates and coldhardiness in terrestrial insects. Eur. J. Entomol. 102(4):561-576.
[https://doi.org/10.14411/eje.2005.081]

- Tabebi K, Hosseininaveh V, Ghadamyari M, 2015. Ecological impacts of pesticides in agricultural ecosystem. pp. 113-168. In: Stoytcheva M (eds). Pesiticde in the modern world : risks and benefits. IntechOpen.
-
Williams CM, Henry HAL, Sinclair BJ, 2015. Cold truths: How winter drives responses of terrestrial organisms to climate change. Bio. Reviews 90(1):214-235.
[https://doi.org/10.1111/brv.12105]

-
Zheng FS, Du YZ, Wang ZJ, Xu JJ, 2008. Effect of temperature on the demography of Galerucella birmanica (Coleoptera: Chrysomelidae). Insect Sci. 15(4):375-380.
[https://doi.org/10.1111/j.1744-7917.2008.00224.x]

In-hong Jeong, Division of Crop Protection, National Institute of Agricultural Sciences, Senior researcher, https://orcid.org/0000-0002-4625-2268
Sung-Wook Jeon, Division of Crop Protection, National Institute of Agricultural Sciences, Postdoctoral researcher, https://orcid.org/0000-0002-5707-4532
Sang-Ku Lee, Division of Crop Protection, National Institute of Agricultural Sciences, Postdoctoral researcher
Si Woo Lee, Department of Vegetable Crops, Korean National University of Agriculture and Fisheries, Senior researcher
Deok Ho Kwon, Department of Vegetable Crops, Korean National University of Agriculture and Fisheries, Professor
Young Ho Koh, Ilsong Institute of Life Sciences, Hallym University, Anyang, Professor, https://orcid.org/0000-0003-3608-4376
Si Hyeock Lee, Department of Agricultural Biotechnology, Seoul National University, Professor
Methodology &Writing-Original Draft Preparation; In-Hong Jeong; Investigation, Sung-Wook Jeon and Sang-Ku Lee; Data Analysis, Young Ho Koh; Writing- Review & Editing, Deok Ho Kwon and Si Woo Lee; Conceptualization & Supervising, Si Hyeock Lee