Fenton Oxidation of Bisphenol A using an Fe3O4-coated Carbon Nanotube: Understanding of Oxidation Products, Toxicity and Estrogenic Activity
Abstract
This study focused on Fenton oxidation of bisphenol A using a Fe3O4-coated multi-walled carbon nanotube (Fe3O4/MWCNT), from which oxidation products, acute toxicity and estrogenic activity were assessed. The final effluents showed no biological toxicity to 24 h born Daphnia magna and negligible in-vivo estrogenic activity. The major oxidation productions generated from heterogeneous Fenton oxidation of bisphenol A included 4-hydroxyacetophenone, 4-hydroxybenzoic acid, catechol and hydroquinone. Therefore, Fenton oxidation of bisphenol A using Fe3O4/MWCNT with a low dose of H2O2 led to high removal of bisphenol A with negligible aquatic toxicity and estrogenic activity. It would be a cost-effective solution for treatment and reuse of emerging contaminant-containing wastewater and water.
Keywords:
Biotoxicity, Estrogenic activity, Bisphenol A, Carbon nanotube, Fe3O4, Advanced oxidationIntroduction
Bisphenol A (BPA), as a well-known endocrine disrupting compound, has been widely used in various industries for past decades (Staples et al., 1998; Rivas et al., 2009; Mboula et al., 2013). Although BPA has been detected at trace levels in wastewater, surface water, landfill leachate and drinking water (Suzuki et al., 2004; Liu et al., 2009; Mohapatra et al., 2010; Xiao et al., 2012), BPA at dilute concentrations can cause hormonal disruption and various cancers (Alexander et al., 1988; Ike et al., 2002; Kuruto-Niwa et al., 2002; Gultekin and Ince 2007; Kim et al., 2007; Pant and Deshpande 2012; Xiao et al., 2012). Thus, effective remediation technologies need to be developed to eliminate BPA from wastewater and water.
Various methods including biodegradation, advanced oxidation and adsorption have been studied for removal of BPA from various wastewater and water (Torres-Palma et al., 2007; Mohapatra et al., 2010; Park et al., 2010; Torres-Palma et al., 2010). Fenton oxidation using H2O2 and iron catalysts among various methods was found to be a highly efficient and cost-effective method for treating BPA (Katsumata et al., 2004; Ioan et al., 2007). However, homogenous Fenton oxidation have the limitations such as low efficiency at neutral pH and generation of excessive iron sludge after reactions (Catrinescu et al., 2003; Pignatello et al., 2006; Diya'uddeen et al., 2012). In order to enhance efficiency of the homogeneous Fenton oxidation heterogeneous Fenton oxidation have been developed by immobilization of iron oxides and catalysts onto various carbon supports (Walling, 1975; Valentine and Wang 1998; Feng et al., 2004; Hsueh et al., 2006; Lim 2006; Oliveira et al., 2007; Hanna et al., 2008).
Recently we prepared the Fe3O4-coated multi-walled carbon nanotube (Fe3O4/MWCNT, hereafter) for effective Fenton oxidation of BPA in water (Cleveland et al., 2014). The Fenton oxidation of BPA using Fe3O4/MWCNT has demonstrated high removal of BPA and COD (i.e., 97-100 % for BPA, 8-60% for Chemical Oxygen Demand) with the [H2O2] : [BPA] of 4 to 10 (mol H2O2/mol BPA) (Cleveland et al., 2014).
However, acute toxicity and estrogenic activity of BPA oxidation products in the treated BPA solution have not been investigated despite these activities are very important for safe and effective treatment, and reuse of treated water. To the best of our knowledge, no studies have been done to assess the aquatic toxicity and the estrogenic activity of BPA oxidation products generated by the Fe3O4/MWCNT-driven heterogeneous Fenton oxidation.
Therefore, the objective of the present study was to assess acute toxicity and estrogenic activity of BPA oxidation products generated by the Fe3O4/MWCNT-driven heterogeneous Fenton oxidation. Specifically, the acute toxicity using Daphnia magna and in-vivo endogenic activity of BPA oxidation products from the heterogeneous Fenton oxidation of BPA were evaluated. The oxidation products were also identified.
Material and Methods
Chemicals and reagents
Multi-walled carbon nanotubes (MWCNT, inside diameter of 5-10 nm and an outside diameters of 60-100 nm with a length of 0.5-500 μm, surface area of 40-300 m2/g ) (Kumar and Mohan 2012), BPA, ferrous sulfate (FeSO4) and hydrogen peroxide were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Acute toxicity of BPA oxidation products
The Fe3O4/MWCNT was prepared by the chemical oxidation followed by co-precipitation as described by Cleveland et al. (2014). The Fenton oxidation was conducted in a glass flask containing 100 mL DI water with 70 mg L−1 (0.31 mM) BPA at the selected conditions (molar ratio of [H2O2] : [BPA] of 4, pH 3 and 50 mg Fe3O4/MWCNT). The pH of aqueous solution for the Fenton oxidation was adjusted with 1 N NaOH and 1 N HCl. These conditions were chosen as the optimum conditions as reported by Cleveland et al. (2014). Although heterogeneous Fenton oxidation would occur at broad ranges of pH, our previous study (Cleveland et al., 2014) indicated highest BPA removal efficiencies at pH 3 which was used for this study. Besides, there was negligible leaching of iron from the Fe3O4 in the carbon nanotube during the Fenton oxidation because of low concentration of H2O2 during for the Fenton oxidation.
The final effluent after 4 h reaction was used for the toxicity tests after the pH of final effluent was adjusted to pH 7. The toxicity of BPA and its oxidation products in the final effluent generated by the Fenton oxidation at the above conditions was tested using 24 h born Daphnia magna at various dilutions (Chahbane et al., 2014). This protocol was based on the procedure as Organization for Economic Cooperation and Development (OECD) Guideline 202. The acute toxicity tests were conducted with five Daphnia magna in each glass flask containing 50 mL of reaction solution with various dilution ratios. Then the Daphnia magna species were cultivated with the final effluent in an incubator at 20oC in a light/dark cycle (16 h/8 h) for 24-48 h. The toxicity was determined as immobilization % obtained by counting the number of survived Daphnia magna after 24 and 48 h.
In-vivo endogenic activity of BPA oxidation products
The final effluent generated by the Fenton oxidation which was used for the biotoxicity test was also used for the in-vivo estrogenic activity of BPA oxidation products. To measure the estrogenic activity of BPA oxidation products, the MCF7-BUS cell line (estrogen-sensitive human breast cancer cells), was kindly provided by Dr. Ana Soto (Tufts University, Boston, MA, USA). The cells were grown in Dulbecco’s Modified Eagle Medium (DMEM; Gibco BRL, Grand Island, NY, USA) supplemented with 5% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), penicillin (100 units/ml) and streptomycin (100 μg/ml) at 37ºC in an atmosphere of 5% CO2/95% air under saturating humidity. The E-screen assay using the MCF7-BUS cells was conducted to identify the estrogenic activity of the BPA solution without Fenton oxidation (70 mg/L) and the BPA solution treated by Fe3O4/ MWCNT-driven Fenton oxidation at the conditions (molar ratio of [H2O2] : [BPA] of 4, pH 3 and 50 mg Fe3O4/MWCNT). Charcoal dextran-treated FBS (CDFBS) was prepared as described (Min et al., 2012) and stored at -20ºC. The cells were harvested with 0.05% trypsin-0.53 mM EDTA·4Na and resuspended in 5%-FBS DMEM. They were seeded onto 48-well plate at a density of 5 × 103 cells/well and incubated for 48 hours. The cells were then treated with 10%-CDFBS DMEM containing three doses of sample A and B (6, 60 and 600 nM) in triplicate. After incubating the cells for 6 days, sulforhodamine B assay was carried out to evaluate the extent of cell proliferation. The relative proliferation effect (RPE) was calculated using the following equation.
RPE = [(S – 1) / (E – 1)] × 100
where S = proliferation of the samples and E = proliferation of positive control (10-10 M E2)
The results of each assay were expressed as mean ± standard deviation. Differences between groups were assessed by one-way ANOVA followed by Duncan’s post-hoc test. Statistical significance was accepted at p < 0.01.
Identification and quantification of BPA and its oxidation products
Chromatographic separation was performed with an AB Sciex triple quadrupole 450 LC-Q-MS/MSD (AB Sciex C0., USA) equipped with a binary pump, a vacuum degasser, low carryover autosampler, a thermostatted column compartment and a Mass Hunter data system. Ten microliters of the extract were injected onto a Kinetex C18 column (100 × 2.1 mm, 3 μm, Phenomenex Co., USA). The mobile phases A and B consisted of 2 mM ammonium acetate in water and methanol, respectively. The analysis for BPA, 4-hydroxyacetophenone (4-HAP), catechol and hydroquinone was performed by using the following gradient program: at a flow rate of 700 μL/min the gradient was initially started at 33% methanol and maintained at that level for 4 min. The gradient was then increased to 60% methanol between 4 and 6 min, increased to 95% methanol between 6 and 7 min, decreased to 33% methanol between 7.1 and 9.5 min, and maintained at this percentage between 9.5 and 12 min.
Results and discussion
The TEM and SEM images of the Fe3O4/MWCNT showed deposition of the Fe3O4 particles (100-150 nm) onto the carbon nanotube (Fig. 1). The Fe3O4 in the Fe3O4/MWCNT has an octahedron crystal structure. It also indicates that the Fe3O4 is well deposited on the MWCNT with little aggregation. The XRD patterns of the Fe3O4/MWCNT also present the major components in the Fe3O4/MWCNT is Fe3O4 (Cleveland et al., 2014).
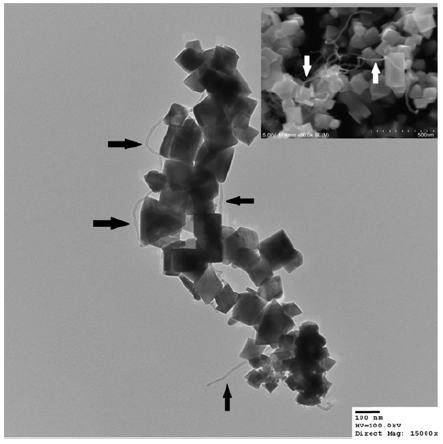
TEM and SEM (inserts) images of the Fe3O4/MWCNT (Cleveland et al., 2014). The arrows indicate MWCNTs.
The heterogeneous Fenton oxidation of BPA using the Fe3O4/MWCNT at the selected conditions (70 mg L−1 BPA, 50 mg Fe3O4/MWCNT, mol H2O2/mol BPA of 4, 20oC, pH 3) was conducted for analyzing the toxicity and estrogenic activity of BPA and its oxidation products. Excellent recoveries (72.5~107.3.4%) were obtained for BPA and its oxidation products such as 4-hydroxyacetophenone (4-HAP), 4-hydroxybenzoic acid (4-HBA), catechol and hydroquinone from the control wastewater (Table 1). The final effluents from the Fenton oxidation of BPA at the above conditions revealed that BPA, 4-HAP, 4-HBA, catechol and hydroquinone were detected at the level of 0.05, 0.06, 0.03, < 0.05 and < 0.05 ng/g in the treated waste water (Table 2). The results in Table 2 are consistent with those from the Fenton oxidation pathways of BPA reported by Poerschmann et al. (2010), Olmez-Hanci et al. (2015), and Hua et al. (2014). Particularly the Fenton oxidation of BPA (i.e., [H2O2]/[BPA] of 2 to 4) made at similar conditions used for the present study, as Poerschmann et al. (2010) reported, indicated that the major oxidation products were 4-HAP, catechol and hydroquinone.
The acute biotoxicity tests using 24 h born Daphnia magna were conducted to estimate aquatic toxicity of the BPA oxidation products in the BPA solution treated by the Fenton oxidation using Fe3O4/MWCNT at the selected conditions (70 mg L−1 BPA, 50 mg Fe3O4/MWCNT, mol H2O2/mol BPA of 4, 20oC, pH 3) (Table 3). The results showed that any immobility of Daphnia magna were not observed from the negative control (the DI water as a sample) and the BPA solution treated by Fenton oxidation using Fe3O4/MWCNT after 24 h and 48 h incubation (Table 3). This supported no biological toxicity of the BPA intermediates and products in the treated BPA solution at the selected conditions since the oxidation products of BPA stayed at 0.03-0.06 mg/L in the aqueous solution. These results are similar to our previous investigation to demonstrate negligible aquatic toxicity in the BPA solution treated by Fe3O4/MWCNT-driven Fenton oxidation using Toxi-ChromotestTM (Environmental Biodetection Products Inc., Mississauga, Canada) (Cleveland et al., 2014). The assessment of biotoxicity using Toxi-ChromotestTM relied on the measurement of toxicant-based inhibition of β-galactosidase synthesis by E. coli as described in by Schalie et al. (2006). Therefore, the Fenton oxidation of BPA using the Fe3O4/MWCNT led to the effective treatment of BPA in wastewater and water. It would contribute to protection of various water resources and helping reuse of wastewater for agricultural and industrial application.
The in-vivo estrogenic activity tests also showed negligible activities from the BPA solution treated by Fe3O4/MWCNTdriven Fenton oxidation at the selected conditions (70 mg L−1 BPA, 50 mg Fe3O4/MWCNT, mol H2O2/mol BPA of 4, 20oC, pH 3) as seen in Fig. 2. The proliferative effects of the BPA solution without Fenton oxidation (70 mg L−1) and the BPA solution treated by Fe3O4/MWCNT-driven Fenton oxidation on MCF7-BUS cells were examined for determining their estrogenic activities. The effects of compounds relative to E2 (10-10 M), which showed the maximum cell proliferative effects, represented as RPE. The frequency of proliferation increased dose-dependently in the BPA solution (untreated by the Fenton oxidation)-treated MCF7-BUS cells, whereas it did not occur in the BPA solution (degraded by the Fenton oxidation)-treated cells (Fig. 2). These results in Fig. 2 supported that the heterogeneous Fenton oxidation of BPA using the Fe3O4/MWCNT resulted in the negligible estrogenic activity. There were a few of publication to report the estrogenic activity of BPA solution treated by advanced oxidation techniques including Fenton oxidation and photochemical oxidation. As reported by Olmez-Hanci et al. (2015), H2O2/ultraviolet light (UV-C) and S2O82−/ultraviolet light (UV-C) processes with high dose of H2O2 showed the complete elimination of estrogenic activity originally retained in the BPA solution (20 mg L−1) when the estrogenic activity was evaluated using the Yeast Estrogen Screen method. On the contrary, the untreated BPA solution and UV-C treated BPA solution did present the clear estrogenic activity (Olmez-Hanci et al., 2015). Compared with the estrogenic results using the above methods (UV-C, H2O2/UV-C and S2O82−/UV-C) requiring high energy consumption and chemical dose the heterogeneous Fenton oxidation of BPA at low dose of H2O2 in this study clearly benefit cost effective and safe remediation while almost completely eliminating biotoxicity and estrogenic activity.
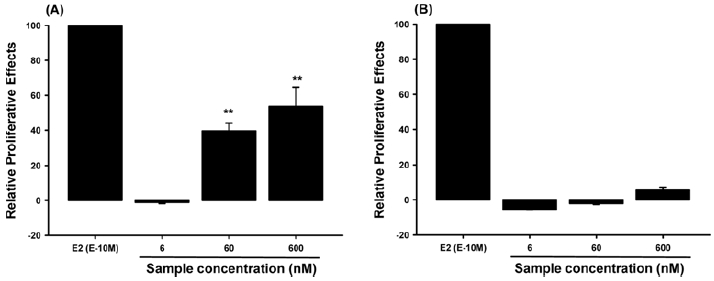
Effects of BPA solution without Fenton oxidation (70 mg/L, A) and BPA solution treated by the MWCNT/ Fe3O4-driven Fenton oxidation (B) on the MCF7-BUS cell proliferation. E2 indicates 17β-estradiol (E2, estrogen) as a positive control. The negative control (the buffer solution) did not show any estrogenic activity. The results of each assay are expressed as mean ± standard deviation (one-way ANOVA followed by Duncan’s post-hoc test. **p < 0.01).
In conclusion, the oxidation products, acute toxicity and estrogenic activity for heterogeneous Fenton oxidation of bisphenol A using Fe3O4/MWCNTs were assessed when the Fenton oxidation occurred at the selected conditions (70 mg L−1 BPA, 50 mg Fe3O4/MWCNT, mol H2O2/mol BPA of 4, 20oC, pH 3). The final effluents from the Fenton oxidation of BPA at the selected conditions showed no biological toxicity when it was tested with 24 h born Daphnia magna. It also resulted in negligible estrogenic activity when in-vivo estrogenic activity of the BPA oxidation products was evaluated. The major oxidation productions generated from the Fenton oxidation of BPA were 4-hydroxyacetophenone (4-HAP), 4-hydroxybenzoic acid (4-HBA), catechol and hydroquinone Therefore, the Fenton oxidation of bisphenol A using Fe3O4/MWCNT with low dose of H2O2 led to high removal of BPA with negligible biotoxicity and estrogenic activity.
Acknowledgments
This work was mainly supported by the “Research Program for Agricultural Science & Technology Development (PJ010896)” at the Rural Development Administration, Republic of Korea. A part of this work was also supported by U.S. Department of Agriculture (Project number: HAW05024-H).
Literature Cited
-
Alexander, H. C, D. C. Dill, L. W. Smith, P. D. Guiney, and P. Dorn, (1988), Bisphenol A: acute aquatic toxicity, Environmental toxicology and chemistry, 7(1), p19-26.
[https://doi.org/10.1897/1552-8618(1988)7[19:baaat]2.0.co;2]

-
Catrinescu, C, C. Teodosiu, M. Macoveanu, J. Miehe-Brendle, and R. Le Dred, (2003), Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite, Water Research, 37(5), p1154-1160.
[https://doi.org/10.1016/s0043-1354(02)00449-9]

- Chahbane, N, D-L. Popescu, D. A. Mitchell, A. Chanda, D. Lenoir, A. D. Ryabov, K-W. Schramm, and T. J. Collins, (2007), FeIII-TAML-catalyzed green oxidative degradation of the azo dye Orange II by H2O2 and organic peroxides: products, toxicity, kinetics, and mechanisms, Green Chemistry, 9(1), p49-57.
-
Cleveland, V, J-P. Bingham, and E. Kan, (2014), Heterogeneous Fenton degradation of bisphenol A by carbon nanotubesupported Fe3O4, Separation and Purification Technology, 133(0), p388-395.
[https://doi.org/10.1016/j.seppur.2014.06.061]

-
Diya'uddeen, B.H, A. R. Abdu, and W. M. A. W. Daud, (2012), On the limitation of fenton oxidation operational parameters: A review, International Journal of Chemical Reactor Engineering, 10(1), p1-12.
[https://doi.org/10.1515/1542-6580.2913]

-
Feng, J., X. Hu, and P. L. Yue, (2004), Novel bentonite clay-based Fe-nanocomposite as a heterogeneous catalyst for photo-Fenton discoloration and mineralization of Orange II, Environmental Science & Technology, 38(1), p269-275.
[https://doi.org/10.1021/es034515c]

- Gultekin, I., and N. H. Ince, (2007), Synthetic endocrine disruptors in the environment and water remediation by advanced oxidation processes, Journal of Environmental Management, 85(4), p816-832.
-
Hsueh, C-L, Y-H. Huang, C-C. Wang, and C-Y. Chen, (2006), Photoassisted Fenton degradation of nonbiodegradable azodye (Reactive Black 5) over a novel supported iron oxide catalyst at neutral pH, Journal of Molecular Catalysis A: Chemical, 245(1), p78-86.
[https://doi.org/10.1016/j.molcata.2005.09.044]

-
Hanna, K, T. Kone, and G. Medjahdi, (2008), Synthesis of the mixed oxides of iron and quartz and their catalytic activities for the Fenton-like oxidation, Catalysis Communications, 9(5), p955-959.
[https://doi.org/10.1016/j.catcom.2007.09.035]

-
Hua, Z, W. Ma, X. Bai, R. Feng, L. Yu, X. Zhang, and Z. Dai, (2014), Heterogeneous Fentondegradation of bisphenol A catalyzed by efficient adsorptive Fe3O4/GO nanocomposites, Environmental Science and Pollution Reseasrch, 21, p7737-7745.
[https://doi.org/10.1007/s11356-014-2728-8]

-
Ike, M, M. Y. Chen, C. S. Jin, and M. Fujita, (2002), Acute toxicity, mutagenicity, and estrogenicity of biodegradation products of bisphenol-A, Environmental toxicology, 17(5), p457-461.
[https://doi.org/10.1002/tox.10079]

-
Ioan, I, S. Wilson, E. Lundanes, and A. Neculai, (2007), Comparison of Fenton and sono-Fenton bisphenol A degradation, Journal of Hazardeous Materials, 142, p559-563.
[https://doi.org/10.1016/j.jhazmat.2006.08.015]

-
Kuruto-Niwa, R, Y. Tera, and R. Nozawa, (2002), Identification of estrogenic activity of chlorinated bisphenol A using a GFP expression system, Environmental Toxicology and Pharmacology, 12(1), p27-35.
[https://doi.org/10.1016/s1382-6689(02)00011-x]

-
Katsumata, H, S. Kawabe, S. Kaneco, T. Suzuki, and K. Ohta, (2004), Degradation of bisphenol A in water by the photo-Fenton reaction, Journal of Photochemistry and Photobiology A: Chemistry, 162(2-3), p297-305.
[https://doi.org/10.1016/s1010-6030(03)00374-5]

-
Kim, Y, K. Choi, J. Jung, S. Park, P-G. Kim, and J. Park, (2007), Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea, Environment International, 33(3), p370-375.
[https://doi.org/10.1016/j.envint.2006.11.017]

- Kumar, A. K., and S. V. Mohan, (2012), Removal of natural and synthetic endocrine disrupting estrogens by multi-walled carbon nanotubes (MWCNT) as adsorbent: Kinetic and mechanistic evaluation, Separation and Purification Technology, 87, p22-30.
- Lim, H, J. Lee, S. Jin, J. Kim, J. Yoon, and T. Hyeon, (2006), Highly active heterogeneous Fenton catalyst using iron oxide nanoparticles immobilized in alumina coated mesoporous silica, Chemical communications, 4, p463-465.
-
Liu, G, J. Ma, X. Li, Q. Qin, (2009), Adsorption of bisphenol A from aqueous solution onto activated carbons with different modification treatments, Journal of Hazardous Materials, 164(2), p1275-1280.
[https://doi.org/10.1016/j.jhazmat.2008.09.038]

-
Mohapatra, D. P, S. K. Brar, R. D. Tyagi, and R. Y. Surampalli, (2010), Physico-chemical pre-treatment and biotransformation of wastewater and wastewater Sludge--Fate of bisphenol A, Chemosphere, 78(8), p923-941.
[https://doi.org/10.1016/j.chemosphere.2009.12.053]

-
Min, C. R, M. J. Kim, Y. J. Park, H. R. Kim, S. Y. Lee, K. H. Chung, and S. M. Oh, (2012), Estrogenic effects and their action mechanism of the major active components of party pill drugs, Toxicology Letters, 214(3), p339-347.
[https://doi.org/10.1016/j.toxlet.2012.09.014]

- Mboula, V. M., V. Hequet, Y. Andree, L. M. Pastrana-Martinez, J. M. Dona-Rodriguez, A. M. T Silva, and P. Falaras, (2013), Photocatalytic degradation of endocrine disruptor compounds under simulated solar light, Water Research, 47, p3997-4005.
-
Oliveira, L, M. Goncalvesm, M. Guerreiro, T. Ramalho, J. Fabris, M. Pereira, and K. Sapag, (2007), A new catalyst material based on niobia/iron oxide composite on the oxidation of organic contaminants in water via heterogeneous Fenton mechanisms, Applied Catalysis A: General, 316(1), p117-124.
[https://doi.org/10.1016/j.apcata.2006.09.027]

-
Olmez-Hanci, T, D. Dursun, E. Aydin, I. Arslan-Alaton, B. Girit, L. Mita, N. Diano, D. G. Mita, and M. Guida, (2015), S2O8(2)/UV-C and H2O2/UV-C treatment of Bisphenol A: Assessment of toxicity, estrogenic activity, degradation products and results in real water, Chemosphere 119 Supplement, pS115-S123.
[https://doi.org/10.1016/j.chemosphere.2014.06.020]

-
Pignatello, J. J, E. Oliveros, and A. MacKay, (2006), Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry, Critical Reviews in Environmental Science and Technology, 36(1), p1-84.
[https://doi.org/10.1080/10643380500326564]

-
Park, S-j, S. S. Chin, Y. Jia, and A. G. Fane, (2010), Regeneration of PAC saturated by bisphenol A in PAC/TiO2 combined photocatalysis system, Desalination, 250(3), p908-914.
[https://doi.org/10.1016/j.desal.2009.06.015]

-
Poerschmann, J, U. Trommler, and T. Gorecki, (2010), Aromatic intermediate formation during oxidative degradation of Bisphenol A by homogeneous sub-stoichiometric Fenton reaction, Chemosphere, 79(10), p975-986.
[https://doi.org/10.1016/j.chemosphere.2010.03.030]

- Pant, J, and S. B. Deshpande, (2012), Acute toxicity of Bisphenol A in rats, Indian Journal of Experimental Biology, 50(6), p425-429.
-
Rivas, F. J, A. Encinas, B. Acedo, and F. J Beltran, (2009), Mineralization of bisphenol A by advanced oxidation processes, Journal of Chemical Technology and Biotechnology, 84, p589-594.
[https://doi.org/10.1002/jctb.2085]

-
Staples, C. A, P. B. Dorn, G. M. Klecka, S. T. O'Block, and L. R. Harris, (1998), A review of the environmental fate, effects, and exposures of bisphenol A, Chemosphere, 36, p2149-2173.
[https://doi.org/10.1016/s0045-6535(97)10133-3]

-
Suzuki, T, Y. Nakagawa, I. Takano, K. Yaguchi, and K. Yasuda, (2004), Environmental Fate of Bisphenol A and Its Biological Metabolites in River Water and Their Xeno-estrogenic Activity, Environmental Science & Technology, 38(8), p2389-2396.
[https://doi.org/10.1021/es030576z]

- Schalie, W. H, R. R. James, and T. P. Gargan, (2006), Selection of a battery of rapid toxicity sensors for drinking water evaluation, Biosensors and Bioelectronics, 22(1), p18-27.
- Torres-Palma, R. A, C. Petrier, E. Combet, F. Moulet, and C. Pulgarin, (2007), Bisphenol A Mineralization by Integrated Ultrasound-UV-Iron (II) Treatment, Environmental Science & Technology, 41(1), p297-302.
-
Torres-Palma, R. A, J. I. Nieto, E. Combet, C. Petrier, and C. Pulgarin, (2010), An innovative ultrasound, Fe2+ and TiO2 photoassisted process for bisphenol a mineralization, Water Research, 44(7), p2245-2252.
[https://doi.org/10.1016/j.watres.2009.12.050]

-
Valentine, R. L., and H. A. Wang, (1998), Iron oxide surface catalyzed oxidation of quinoline by hydrogen peroxide, Journal of Environmental Engineering, 124(1), p31-38.
[https://doi.org/10.1061/(asce)0733-9372(1998)124:1(31)]

- Walling, C., (1975), Fenton's reagent revisited, Accounts of Chemical Research, 8(4), p125-131.
-
Xiao, G, L. Fu, and A. Li, (2012), Enhanced adsorption of bisphenol A from water by acetylaniline modified hypercross-linked polymeric adsorbent: Effect of the cross-linked bridge, Chemical Engineering Journal, 191, p171-176.
[https://doi.org/10.1016/j.cej.2012.02.092]

