Dissipation Patterns of Insecticide Sulfoxaflor in Spinach and Korean Cabbage

Abstract
The present greenhouse experiment was carried out to study the residues of sulfoxaflor in spinach and Korean cabbage under different pre-harvest application regimes. After 2 times application of with 1 week interval in that day of harvest (0), 3, 7, 14 and 21 days before harvest, and the residue of pesticides was investigated by LC-MS/MS. Matrix-matched calibration curves for sulfoxaflor in both spinach and Korean cabbage yielded good linearity (R2 ≥ 0.9995). The biological half-lives of sulfoxaflor in spinach and Korean cabbage deduced from first order forms of decline (C=3.2539e−0.179t in spinach and C=1.2432e−0.196t in Korean cabbage) were 3.87 and 3.54 days, respectively. Pre-harvest residues of sulfoxaflor in spinach at 10-3 days (2.16 mg/kg) and 7-0 days (2.97 mg/kg) were above the EU-MRL (2.0 mg/kg), but within the MFDS value of 3.0 mg/kg. In Korean cabbage, residues at 10-3 days (0.74 mg/kg) and 7-0 days (1.17 mg/kg) were above the MFDS value of 0.3 mg/kg, though below the EU-MRL (6.0 mg/kg). Based on field incurred residues, it’s safe to apply sulfoxaflor to spinach as per MFDS regulation and Korean cabbage at 21-14 or 14-7 days before harvest as per EU regulation.
Keywords:
Korean cabbage, LC-MS/MS, Pre harvest residue, Sulfoxaflor, SpinachIntroduction
Spinach and cabbage are popular vegetables grown and consumed worldwide. They have high content of minerals and vitamins and are consumed as boiled, salad, pickles, cooked curries, fermented (“Kimchi”) and dehydrated vegetable (Butnariu & Butu, 2015) and cover the global estimated production of 26.68 and 71.26 million tons respectively; of which Korea reportedly produces these vegetables 0.089 and 2.5 million tons, accordingly (FAOSTAT, 2018). However, production of these important vegetables is constrained by several insect pests, with the major ones being aphids, diamond black moth, root flies, bugs, loopers, leaf miners, cutworms and beetles. In a desperate move to save their crop from insect damage, farmers have made application of pesticides an imperative routine (Gupta et al., 2015).
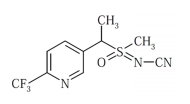
The consequence of the extensive use of pesticides has not only led to many insects developing resistance to most of the commonly used pesticides but a health hazard to humans as well (Mengistie et al., 2017). Therefore, pesticides in newer groups of insecticides like neonicotinoids and sulfoximines are reported to be effective against a broad range of insect pests as they do not exhibit cross-resistance (Nugent et al., 2015). Sulfoxaflor (Table 1), a notable sulfoximine, has particularly been showed to have greater efficacy against a majority of the insect pests, including those displaying crossresistance to many of the insecticides currently in use (Sparks et al., 2013).
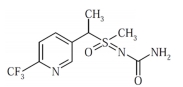
The potency of sulfoxaflor against a broad spectrum of insects such as hoppers, whiteflies and aphids is through its action as a nicotinic acetylcholine receptor (nAChR) competitive modulator (Sparks et al., 2013; Watson et al., 2017). Pesticide use in crop protection often results in residues of parent compounds and/or degradation metabolites. In particular, sulfoxaflor is known to degrade to two metabolites in plants: X11719474 and X11721061, though both of which have been showed to have low mammalian toxicity (FAO and WHO, 2011) The pesticides applied are either absorbed and persist in plants or they reach the soil environment which acts as a sink for further degradation and/ or distribution to other environmental compartments (Arias-Estévez et al., 2008). Residual portions of pesticides parent compounds and/or their degraded metabolites may thus be hazardous to the consumers, and as a consequence, regulation of food commodities treated with pesticides is paramount (Hrouzková et al., 2013).
World over, food commodities treated with pesticides are highly regulated using measurement of pesticide residue levels (Hrouzková et al., 2013; Tsakiris et al., 2015). The maximum residue level (MRL) is the tolerable residue level in any commodity set by authorities in a particular country or regions as a means to minimize pesticides intake for good consumer health (Singh & Singh, 2014; Sabarwal et al., 2018). The MRL for a particular pesticide on a given agricultural product is either set by a country/region or the Codex Alimentarius values are used. In Korea, the Ministry of Food and Drug Services (MFDS) sets MRL values for pesticides on different crops based on their toxicological effects and consumption levels. According to the MFDS, the MRLs for sulfoxaflor are 3.0 mg/kg and 0.3 mg/kg in spinach and Korean cabbage respectively, whereas the European Union Commission, MRLs for sulfoxaflor in Spinach and cabbage are 6.0 mg/kg and 2.0 mg/kg respectively (SANTE, 2018). Once applied to crops, pesticide concentration reduces with time as the pesticide dissipates; and since pesticides are applied following regiments like 21-14; 14-7; 10-3 or 7-0 days before harvest, determining a regiment that results in residues below the pesticide MRL is vital. Due to differences in crop morphology and chemical molecular structure, pesticides retention and spatial concentrations tend to differ across different plants (Buchholz and Trapp, 2016). Pesticide residue analysis is routinely carried out on vegetables and other minor crops for establishing MRLs of pesticides in Korea in previous studies (Lee et al., 2012; Son et al., 2012; Jeon et al., 2015).
Various methods have been reported for analysis of sulfoxaflor residues in agricultural commodities. Primarysecondary amine (PSA) and ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) was used for detection of sulfoxaflor in vegetables, fruits, and soil (Xu et al., 2012). Tian et al., (2016) determined residues of sulfoxaflor in animal-based food using dispersivesolid-phase extraction (d-SPE), a multi -plug filtration cleanup method and UPLC–MS/MS. Several earlier attempts made successful for analysis of pesticides in minor crops in Korea by using modification of QuEChERS techniques (Lee et al., 2012; Kim et al., 2017). In this study, sulfoxaflor and its metabolites were determined using QuEChERS extraction and clean-up with primary-secondary amine (PSA) followed by LC-MS/MS analysis.
For safety purposes therefore, establishing application regiments that result in minimal pre-harvest residues of either pesticide parent compounds and/or the metabolites is vital for consumer safety. Therefore, the current study is aimed to establish a safe pre-harvest day of sulfoxaflor application by maintaining the residue level below the MRL.
Materials and methods
Chemicals and reagents
High grade sulfoxaflor and its metabolites (X11719474 and X11721061) of purity > 99% were obtained from SigmaAldrich Co. (Korea). High performance liquid chromatography (HPLC) grade acetonitrile (MeCN) and water were obtained from Honeywell – Burdick and Jackson Inc. (USA). Analytical-grade sodium chloride (NaCl) was obtained from Junsei Chemical Co. Ltd (Japan). PSA was obtained from Agilent technologies (USA). Commercial type of Sulfoxaflor 7% Suspension Concentrate (SC) was obtained from Farm Hannong Co., Ltd. (Korea).
Standard solutions
Standard stock solutions of sulfoxaflor and its metabolites X11719474 and X11721061 were individually prepared in MeCN at the concentration of 500 mg/L. These were stored at –20oC. For preparation of calibration curves, working solutions were prepared by serially diluting the stock solutions in the same solvent (MeCN). Matrix-matched concentrations of 0.1, 0.2, 0.5, 1.0, 2.0, 5.0 and 10.0 ng/L for sulfoxaflor and metabolites were prepared in spinach and Korean cabbage blank extracts. Standard solutions were cryo-preserved (–20oC) throughout the analysis period.
Field experiment
A greenhouse field test was conducted in the experimental farm located in Waegwan-eup, Chilgok-gun, Gyeongsangbukdo, Republic of Korea, between February 26th 2018 (Planting) and April, 4th 2018 (Harvest). The experimental site consisted of greenhouse covering an area of 480 m2 and was divided into four plots (120 m2 each). Two of the plots were planted with spinach, while the other two with Korean cabbage. For each crop, one plot was selected for pesticide application while the others served as controls. The plots applied with the pesticide were further subdivided into four sets of 40 m2 to suit the pesticide application regimes. These were further subdivided into smaller units of 5 m × 2 m (10 m2) as replications. Commercial formulation of Sulfoxaflor (7% SC) was applied to experimental plots at a rate of 0.007 kg ai/10a (0.07 kg a.i/ha) using a manual knapsack sprayer. Following a sequenced application pattern of 21-14; 14-7; 10-3 and 7-0 days to harvest, all samples from each experimental unit were harvested and collected on April 4th, 2018. During the experimental period, conditions in the greenhouse were controlled at 6.3-22.6oC and 26.5-72.7% humidity for cultivation of Korean cabbage and 3.2-21.1oC and 26.1-95.5% humidity for cultivation of spinach.
Processing and storing of plant samples
The samples were packed in paper bags and transferred to the analytical laboratory of Environmental chemistry of Kyungpook National University in iced boxes. Samples from each of the replicates constituting an application pattern were mixed to form a homogenous sample of about 2.5 kg. It was blended for sufficient mixing by homogenizer, placed into zip lock storing bags and stored at –20oC prior to analysis as previously described (Hwang et al., 2018a).
Sample preparation for chromatography
Samples were prepared using a modified QuEChERS method (Lee et al., 2012; Chung et al., 2017; Kim et al., 2017). Briefly, 10 g of blended samples of Korean cabbage and spinach were weighed into 50 mL Teflon centrifuge tubes using laboratory scale (XB 3200C Precisa Ltd. Switzerland). 20 mL of acetonitrile was added to the tubes and vortex shaken for 20 minutes at 2,500 rpm (Talboys Advanced Multi-tube vortexer). 1 g of NaCl was then added and vortex shaken for 1 minute at 2,500 rpm before centrifuging at 4,000 rpm for 5 minutes (Hanil MF 300 centrifuge, Korea). 2 mL of the supernatant was transferred to a 15 mL Teflon centrifuge tube where 50 mg of PSA had been added. The tubes were shaken for one minute at 2,500 rpm (Talboys Advanced Multi-tube vortexer, Henry Troemner LLC, USA) before being centrifuged for 5 minutes at 4,000 rpm (Hanil MF 300, centrifuge, Korea). 2 mL of the supernatant was transferred to 2 mL auto-sampler vials through 0.2 μm nylon membrane filters (Whatman GmbH, Germany) before analysis by LC-MS/MS.
LC-MS/MS Instrumental conditions
The LC-MS/MS analysis system consisted of a Shimadzu LC-MS 8030 with Nexera UFLC coupled to triple quadrupole tandem mass (Shimadzu Scientific Inst. Inc., Japan) equipped with electron spray ionization (ESI) source. A Capcell PAK C18 MG 100 Å 3 μm column (150 × 3.0 mm, Shiseido Co., Japan) was used for separating analytes injected in at 10 μL. A mobile phase comprising of a binary solvent system of 0.1% formic acid in methanol (A) and 0.1% formic acid in water (B) was used in a gradient mode with a flow rate of 0.3 mL/min. The mobile phase starting composition was 1% A (0 – 3 min), ramped to 37% A (3 – 6 min), held at 37% A (6 – 10 min), decreased to 1% A (10 – 13 min), then was maintained at 1% A (13 – 15 min).
The mass spectrometry (MS) conditions were: capillary voltage, 2.0 kV; desolvation temperature 250oC; collision gas (Ar), 230 kPa; nebulizing gas flow 3.0 L/min; drying gas flow 15.0 L/min; electrospray mode was. The MS measurements were performed in positive electrospray ionization (ESI +) mode with multiple reaction monitoring (MRM) and details of MRM parameters are noted in Table 2. Data acquisition/processing and instrumental control were done using LabSolutions LCMS Version 5.4 for LCMS-8030 software.
Quality control
In this study, the residual levels of sulfoxaflor in plants was expressed as the total residual levels, and the total residual levels were calculated as the sum of parent compound and metabolites X11719474 and X11721061 shown in the following formula. Total residual levels of sulfoxaflor = residual levels of sulfoxaflor + (residual levels of X11721061 × 1.451)) + (residual levels of X11719474 × 0.942))
| 1) |
| 2) |
Validation of the analytical method was done by evaluating linearity, specificity, recovery, and limit of detection (LOD) and quantification (LOQ). Linearity (R2) was assessed using matrix matched calibration curves created using seven points with different concentrations (0.1, 0.2, 0.5, 1.0, 2.0, 5.0 and 10.0 mg/L). The LOD was set at 0.02 mg/kg. Specificity was evaluated by comparing blank and standard chromatograms for absence of any interference around the standard retention time. Recovery was evaluated by fortifying blank samples of spinach and Korean cabbage at two concentration levels 2 mg/kg (10 × LOQ) and 10 mg/kg (50 × LOQ) in three replicates. Precision was expressed as relative standard deviation (RSD, %).
Dissipation of Sulfoxaflor
Pesticide dissipation is commonly determined as a measure of pesticide concentration over time ( Xu et al., 2012; Kang et al., 2016; Kim et al., 2017). The dissipation of sulfoxaflor in spinach and Korean cabbage was determined by plotting the sum of concentration levels of the parent compound of sulfoxaflor and the two metabolites (X11719474 and X11721061) against time. The rate equations developed were of the form Ct = Coe−kt, where Ct represents the concentration of pesticide at time t, Co represents the initial concentration and k is the rate constant in days−1. The dissipation half-life (t1/2) of sulfoxaflor in both spinach and Korean cabbage were determined using the expression t1/2 = ln2/k.
Data Analysis
MS Excel (MS Office 2010) and Solver add-in of Excel was used to generate rate equation, dissipation graphs and relevant statistical analysis.
Results and discussion
Analysis method validation
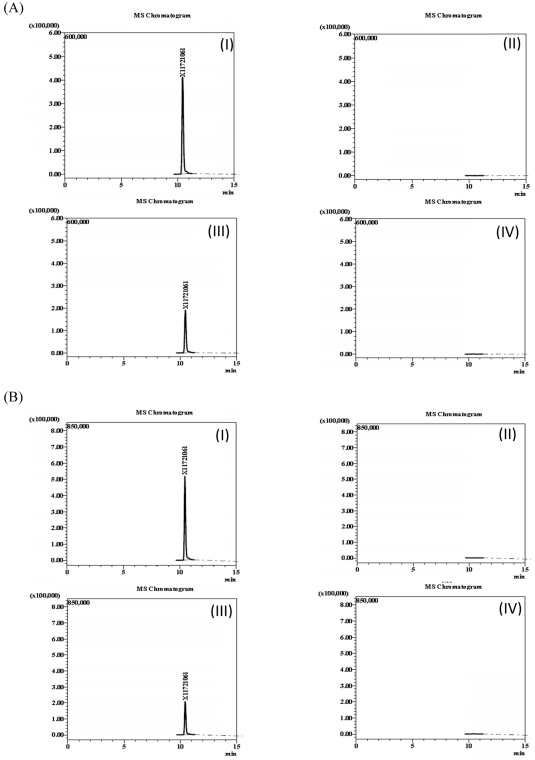
The specificity of the method was assessed by spiking blank samples of spinach and Korean cabbage with sulfoxaflor or its metabolites (X11719474 and X11721061) and comparing with results of samples that did not contain any of the target compounds. The chromatograms of sulfoxaflor (Fig. 1), metabolite X11719474 (Fig. 2) and metabolite X11721061 (Fig. 3) in solvent, fortified and blank samples exhibited absence of interfering peaks at the target compound retention times. Matrix-matched calibration curves linearity was evaluated using a seven level concentration ranging from 0.1 – 10.0 ng/L for both sulfoxaflor and metabolites (Sulfoxaflor, X11719474 and X11721061). Adequate linearity with coefficients of determination R2 ≥ 0.9995 was achieved for all tested analytes.
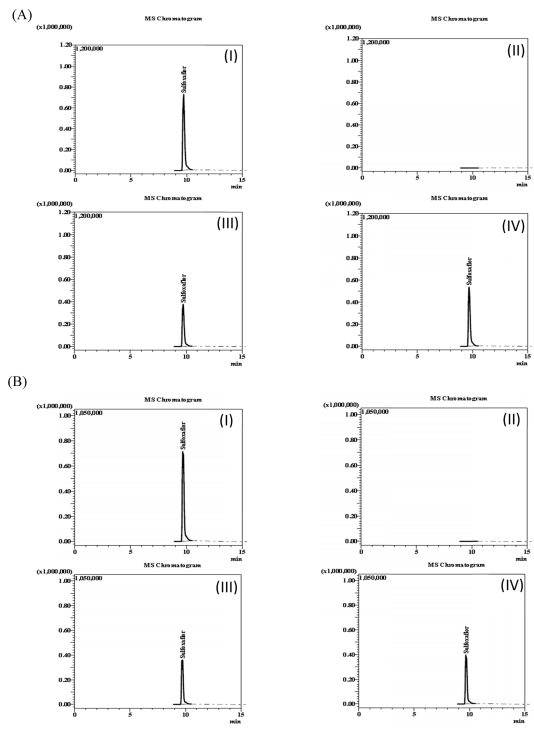
Chromatograms of sulfoxaflor in spinach (A) and Korean cabbage (B), (I) matrix-matched standard (1.0 mg/kg); (II) blanks (control); (III) fortified samples (1.0 mg/kg); and (IV) field-incurred samples (7-0 days).
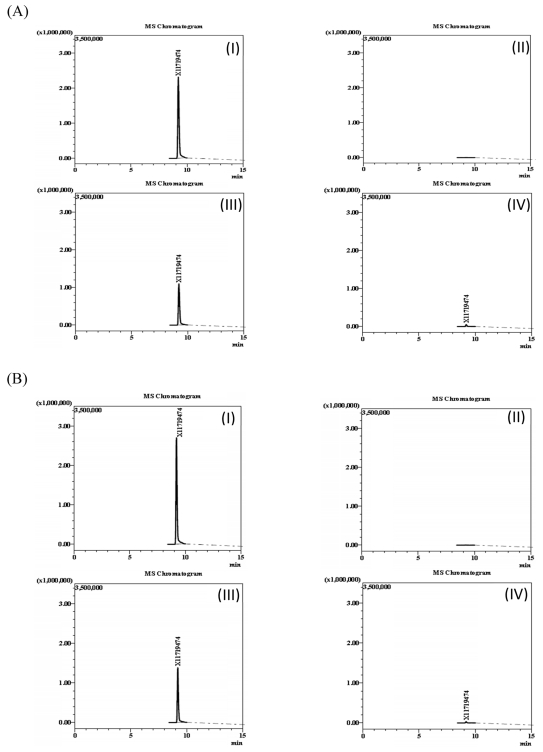
Chromatograms of X11719474 in spinach (A) and Korean cabbage (B), (I) matrix-matched standard (1.0 mg/kg); (II) blanks (control); (III) fortified samples (1.0 mg/kg); and (IV) field-incurred samples (7-0 days).
The recovery of the analytes (Sulfoxaflor, X11719474 and X11721061) were determined in triplicate to evaluate extraction efficiency. The recovery experiment on both spinach and Korean cabbage consisted of two fortification levels (0.2 and 1.0 mg/kg) for both sulfoxaflor and the two metabolites (X11719474 and X11721061) (Table 3). In all cases, good recoveries of analytes were registered and ranged from 89.0% to 107.9% with standard deviation values of 0.7 – 4.2%. These results are consistent with specified acceptable range of 70 – 120% implying good precision of analysis method used in this study.
Sulfoxaflor residues analysis
Residues of Sulfoxaflor in spinach and Korean cabbage sprayed two times at different pre-harvest days were determined after conducting recovery tests. Table 4 shows residual levels of sulfoxaflor and its metabolites in spinach and Korean cabbage samples. Maximum residues were recorded in samples sprayed at 7-0 days before harvest (2.97 mg/kg and 1.19 mg/kg in spinach and Korean cabbage, respectively) and 10-3 days (2.16 mg/kg and 0.74 mg/kg in spinach and Korean cabbage, respectively). These residual levels were above the MRLs (2.0 mg/kg) for spinach, and 0.3 mg/kg in Korean cabbage. Overall, residual levels of sulfoxaflor substantially reduced with time following these pre-harvest application regimes (Kim et al., 2017). Morphologically, Korean cabbage has high water mass which is expected to offer more dilution to the pesticide and hence resulting in generally lower concentrations. These results are consistent with early research results (Hanafi et al., 2010; Lee et al., 2012; Bletsou et al., 2013) on the effects of plant shape on pesticide residues. The implication of this is that at the same application rates, sulfoxaflor would dissipate faster in Korean cabbage than spinach.
Dissipation of sulfoxaflor in spinach and Korean cabbage
The dissipation of sulfoxaflor in spinach and Korean cabbage was of exponential nature and corresponded to a first order kinetics.
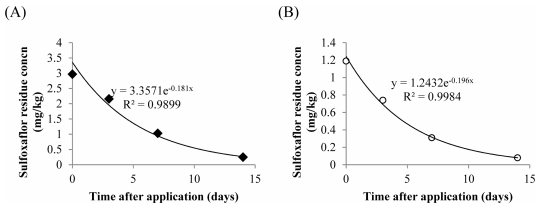
Initial concentration of sulfoxaflor in spinach (2.97 mg/ kg), was higher than that in Korean cabbage (1.17 mg/kg), despite the similarity in application rate (0.07 kg a.i. /ha). This may be due to differences in their physiology and growth habits. Furthermore, Korean cabbage is expected to offer more dilution effect to the pesticide as compared to spinach owing to more water held in its thick tissue mass. It is noted here that the half-lives of sulfoxaflor in the two crops, that is Korean cabbage (3.54 days) and spinach (3.83 days) closely correlates with previous studies (Table 5). Previous studies report the biological half-life of sulfoxaflor in cucumber to be in the range of 1.6-2.9 days (Chen et al., 2016) and 1.5 days in greenhouse lettuce (Kim et al., 2017). The concentration of the pesticides at any given time could be determined using the dissipation equations C = 3.3571e−0.181t for spinach, and C = 1.2432e−0.196t for Korean cabbage (Fig. 4).
Pre-harvest residues of sulfoxaflor
The MRL of sulfoxaflor for spinach recommended by the EU and MFDS are 2.0 mg/kg and 3.0 mg/kg, respectively. Among the pre-harvest application treatments, 21-14 days and 14-7 days before harvest showed lower total residues of 0.25 mg/kg and 1.03 mg/kg respectively (Table 4). Preharvest residues of sulfoxaflor in spinach at 10-3 days (2.16 mg/kg) and 7-0 days (2.97 mg/kg) were above the EU-MRL (2.0 mg/kg), but within the MFDS value of 3.0 mg/kg. In Korean cabbage, residues at 10-3 days (0.74 mg/kg) and 7-0 days (1.17 mg/kg) were above the MFDS value of 0.3 mg/ kg, though below the EU-MRL (6.0 mg/kg). Based on field incurred residues, it’s safe to apply sulfoxaflor to spinach as per MFDS regulation and Korean cabbage at 21-14 or 14-7 days before harvest as per EU regulation. The finding of the present study is in line with previous studies on pre-harvest intervals (Kim et al., 2014; Hwang et al., 2018b).
Conclusions
In summary, the residue patterns of sulfoxaflor varied in spinach and Korean cabbage under different pre-harvest application regiments. Comparatively, the residual amount of sulfoxaflor were low in Korean cabbage over the pre-harvest period investigated. Due to the high moisture in the fleshy tissue of Korean cabbage that offers a higher dilution effect of the pesticide residue in plants. Residues of sulfoxaflor in spinach at 10-3 days (2.16 mg/kg) and 7-0 days (2.97 mg/kg) before harvest and in Korean cabbage 10-3 days (0.74 mg/ kg) and 7-0 days (1.19 mg/kg) before harvest were above the MRL. Thus, application of sulfoxaflor on spinach or Korean cabbage at 10-3 and 7-0 days before harvest is not safe for consumers. Therefore, the application regimes of 21-14 days and 14-7 days before harvest will be recommended. With regard to terminal residues attributed dissipation and biological half-lives of sulfoxaflor in spinach (3.83 days) and Korean cabbage (3.54 days). The dissipation rate equations for sulfoxaflor in spinach and Korean cabbage can be used to compute pre-harvest residues of sulfoxaflor in either of these crops at any point time and hence ensure safe application.
Acknowledgments
This research was supported by a grant (00-18-8-056300) from Ministry of Food and Drug Safety in 2018.
References
-
Arias-Estévez, M., López-Periago, E., Martínez-Carballo, E., Simal-Gándara, J., Mejuto, J.-C., and L. García-Río, (2008), The mobility and degradation of pesticides in soils and the pollution of groundwater resources, Agri. Eco. Environ, 123(4), p247-260.
[https://doi.org/10.1016/j.agee.2007.07.011]

-
Bletsou, A. A., A. H. Hanafi, M. E. Dasenaki, and N. S. Thomaidis, (2013), Development of Specific LC-ESI-MS/MS Methods to Determine Bifenthrin, Lufenuron, and Iprodione Residue Levels in Green Beans, Peas, and Chili Peppers Under Egyptian Field Conditions, Food Anal. Methods, 6(4), p1099-1112.
[https://doi.org/10.1007/s12161-012-9515-2]

-
Buchholz, A., and S. Trapp, (2016), How active ingredient localisation in plant tissues determines the targeted pest spectrum of different chemistries: Intracellular localisation of insecticides, Pest Mgt. Sci, 72(5), p929-939.
[https://doi.org/10.1002/ps.4070]

-
Butnariu, M., and A. Butu, (2015), Chemical Composition of Vegetables and Their Products, In Handbook of Food Chemistry, P. C. K. Cheung, and B. M. Mehta (Eds.), Berlin, Heidelberg, Springer Berlin Heidelberg., p627-692.
[https://doi.org/10.1007/978-3-642-36605-5_17]

-
Chen, Z., F. Dong, X. Pan, J. Xu, X. Liu, X. Wu, and Y. Zheng, (2016), Influence of Uptake Pathways on the Stereo selective Dissipation of Chiral Neonicotinoid Sulfoxaflor in Greenhouse Vegetables, J. Agril Food Chem, 64(13), p2655-2660.
[https://doi.org/10.1021/acs.jafc.5b05940]

-
Chung, H. S., A. M. Abd El-Aty, S. W. Kim, H. S. Lee, M. Rahman, H. Kabir, H. C. Shin, and J. H. Shim, (2017), Simultaneous determination of sulfoxaflor and its metabolites, X11719474 and X11721061, in brown rice and rice straw after field application using LC-MS/MS, Int. J. Environ. Ana. Chem, 97(2), p99-111.
[https://doi.org/10.1080/03067319.2017.1282473]

- FAO Panel of Experts on Pesticide Residues in Food and the Environment, & WHO Expert Group on Pesticide Residues (FAO and WHO), (2011), Pesticide residues in food 2011, joint FAO/WHO meeting on pesticide residue, Geneva, Switzerland, 20-29 September 2011.
- FAOSTAT, (2018), FAOSTAT Crop production data, Food and Agril, Org of UN, Retrieved from http://www.fao.org/faostat/en/#data/QC.
-
Gupta, S., R. K. Sharma, V. T. Gajbhiye, and R. K. Gupta, (2015), Residue behavior of combination formulations of insecticides in/on cabbage and their efficacy against aphids and diamondback moth, Environ. Monit. Assess, 187(1), p4076.
[https://doi.org/10.1007/s10661-014-4076-z]

-
Hanafi, A., V. L. Garau, P. Caboni, G. Sarais, and P. Cabras, (2010), Minor crops for export: A case study of boscalid, pyraclostrobin, lufenuron and lambda-cyhalothrin residue levels on green beans and spring onions in Egypt, J. Environ. Sci. Health, Part B, 45(6), p493-500.
[https://doi.org/10.1080/03601234.2010.493466]

-
Hrouzková, S., M. Andraš íková, S. B. A. Ghani, and A. Purdešová, (2013), Investigation of Levels and Fate of Pyridalyl in Fruit and Vegetable Samples by Fast Gas Chromatography-Mass Spectrometry, Food Anal. Methd, 6(3), p969-977.
[https://doi.org/10.1007/s12161-012-9508-1]

-
Hwang, J. I., H. Y. Kim, S. H. Lee, S. Y. Kwak, A. R. Zimmerman, and J. E. Kim, (2018b), Improved dissipation kinetic model to estimate permissible pre-harvest residue levels of pesticides in apples, Env. Monit. Assess, 190(7), p438.
[https://doi.org/10.1007/s10661-018-6819-8]

-
Hwang, J. I., A. R. Zimmerman, and J. E. Kim, (2018a), Bioconcentration factor-based management of soil pesticide residues: Endosulfan uptake by carrot and potato plants, Sci. Total Environ, 627, p514-522.
[https://doi.org/10.1016/j.scitotenv.2018.01.208]

-
Jeon, S. O., J. I. Hwang, T. H. Kim, C. H. Kwon, Y. U. Son, D. S. Kim, and J. E. Kim, (2015), Residual Patterns of Insecticides Bifenthrin and Chlorfenapyr in Perilla Leaf as a Minor Crop, Kor. J. Environ. Agril, 34(3), p223-229.
[https://doi.org/10.5338/KJEA.2015.34.3.29]

-
Kang, J. G., J. I. Hwang, S. H. Lee, S. O. Jeon, S. Y. Kwak, J. H. Park, and J. E. Kim, (2016), Residual Patterns of Fungicides Fludioxonil and Metconazole in Different Parts of Wheat, Kor. J. Pesti. Sci, 20(4), p341-348.
[https://doi.org/10.7585/kjps.2016.20.4.341]

-
Kim, D. S., K. J. Kim, H. N. Kim, J. Y. Kim, and J. H. Hur, (2014), Determination of Pre-Harvest Residue Limits of Pesticides Metalaxyl-M and Flusilazole in Oriental Melon, Kor. J. Pesti. Sci, 18(1), p1-7.
[https://doi.org/10.7585/kjps.2014.18.1.1]

-
Kim, S. W., M. Rahman, A. M. A. El-Aty, H. Kabir, T. W. Na, J. H. Choi, and J. H. Shim, (2017), Simultaneous detection of sulfoxaflor and its metabolites, X11719474 and X11721061, in lettuce using a modified QuEChERS extraction method and liquid chromatography-tandem mass spectrometry: Residue analysis of sulfoxaflor and its metabolites in lettuce, Biomed. Chrom, 31(6), pe3885.
[https://doi.org/10.1002/bmc.3885]

-
Lee, J. Y., S. M. Hong, T. K. Kim, Z. W. Min, Y. H. Kim, K. A. Song, H. Y. Kwon, H. D. Lee, G. J. Im, D. H. Kim, and J. E. Kim, (2012), Modified QuEChERS Multi-Residue Analysis Method for 61 pesticides in Fruits using with HPLC and GC-ECD/NPD, Kor. J Pesti. Sci, 16(3), p242-256.
[https://doi.org/10.7585/kjps.2012.16.3.242]

-
Mengistie, B. T., A. P. J. Mol, and P. Oosterveer, (2017), Pesticide use practices among smallholder vegetable farmers in Ethiopian Central Rift Valley, Environ. Dev. Sustain, 19(1), p301-324.
[https://doi.org/10.1007/s10668-015-9728-9]

-
Nugent, B. M., A. M. Buysse, M. R. Loso, J. M. Babcock, T. C. Johnson, M. P. Oliver, T. P. Martin, M. S. Ober, N. Breaux, A. Robinson, and Y. Adelfinskaya, (2015), Expanding the structure-activity relationship of sulfoxaflor: the synthesis and biological activity of N-heterocyclic sulfoximines, Pest Mgt. Sci, 71(7), p928-936.
[https://doi.org/10.1002/ps.3865]

-
Sabarwal, A., K. Kumar, and R. P. Singh, (2018), Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders, Environ. Toxicol. Pharm, 63, p103-114.
[https://doi.org/10.1016/j.etap.2018.08.018]

- SANTE, (2018), SANTE/10478/2018, https://prodstoragehoeringspo.blob.core.windows.net/7ad7fb2b-987b-4a3c-93c7-266a8a3593a4/Pt.%20B%2001.00%20Current%20and%20new%20values_10478.pdf.Accessed28September2018.
-
Singh, G., and B. Singh, (2014), Residue Dynamics and Risk Assessment of Trifloxystrobin and Tebuconazole on Tomato (Lycopersicon esculentum Mill.), Amer. J. Environ. Protect, 2(3), p59-63.
[https://doi.org/10.12691/env-2-3-2]

-
Son, K.-A., G. J. Im, S. M. Hong, J. B. Kim, Y. B. Ihm, H. S. Ko, and J. E. Kim, (2012), Comparison of Pesticide Residues in Perilla Leaf, Lettuce and Kale by Morphological Characteristics of Plant, Kor. J. Pesti. Sci, 16(4), p336-342.
[https://doi.org/10.7585/kjps.2012.16.4.336]

-
Sparks, T. C., G. B. Watson, M. R. Loso, C. Geng, J. M. Babcock, and J. D. Thomas, (2013), Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects, Pesti. Biochem. Physiol, 107(1), p1-7.
[https://doi.org/10.1016/j.pestbp.2013.05.014]

-
Tian, C., J. Xu, F. Dong, X. Liu, X. Wu, H. Zhao, C. Ju, D. Wei, and Y. Zheng, (2016), Determination of Sulfoxaflor in Animal Origin Foods Using Dispersive Solid-Phase Extraction and Multiplug Filtration Cleanup Method Based on Multiwalled Carbon Nanotubes by Ultraperformance Liquid Chromatography/Tandem Mass Spectrometry, J. Agril. Food Chem, 64(12), p2641-2646.
[https://doi.org/10.1021/acs.jafc.6b00285]

-
Tsakiris, I. N., M. Goumenou, M. N. Tzatzarakis, A. K. Alegakis, C. Tsitsimpikou, E. Ozcagli, D. Vynias, and A. M. Tsatsakis, (2015), Risk assessment for children exposed to DDT residues in various milk types from the Greek market, Food Chem. Toxicol, 75, p156-165.
[https://doi.org/10.1016/j.fct.2014.11.012]

-
Watson, G. B., M. B. Olson, K. W. Beavers, M. R. Loso, and T. C. Sparks, (2017), Characterization of a nicotinic acetylcholine receptor binding site for sulfoxaflor, a new sulfoximine insecticide for the control of sap-feeding insect pests, Pesti. Biochem. Physiol, 143, p90-94.
[https://doi.org/10.1016/j.pestbp.2017.09.003]

-
Xu, J., F. Dong, X. Liu, J. Li, Y. Li, W. Shan, and Y. Zheng, (2012b), Determination of sulfoxaflor residues in vegetables, fruits and soil using ultra-performance liquid chromatography/tandem mass spectrometry, Anal. Methd, 4(12), p4019.
[https://doi.org/10.1039/c2ay25782c]