Soil Metabolism of [14C]MRC-04 under Aerobic and Aerobic Flooded and Anaerobic Conditions
Abstract
MRC-04 (5-(((2,6-difluorobenzyl)oxy)methyl)-5-methyl-3-(o-tolyl)-4,5-dihydroisoxazole), is an analogue of a new turf herbicide methiozolin, is a herbicide candidate with a potent herbicidal activity to various paddy weeds, especially against susceptible and ALS-resistant barnyardgrass. The fate of MRC-04 was investigated in soil under an aerobic, aerobic flooded, and anaerobic condition using [benzyl-14C]MRC-04, describing the mass balance, the degradation pattern and formation of metabolites. Overall mass balance was in the range of 90.2-103.3% of the applied in all the soil conditions. MRC-04 steadily degraded to 23.6 and 11.7% of the applied at 120 and 60 days, and the estimated half-life was 63.0 and 17.9 days in the soil under the aerobic and aerobic flooded conditions, respectively. However, the half-life could not be calculated in the soil under the anaerobic condition because MRC-04 degradation was limited. Carbon dioxide increased to 24.1, 30.9 and 13.7% of the applied, and non-extractable residue increased to 35.9, 11.6, and 15.3% at 120, 60, and 120 days in the soil under the aerobic, aerobic flooded, and anaerobic conditions, respectively. No significant volatile compound was detected during this study. Three major metabolites were detected in the soil under aerobic flooded condition and identified as 2,6-difluorobenzoic acid, 4-((2,6-difluorobenzyl)oxy)-3-hydroxy-3-methyl-1-(o-tolyl)butan-1-one and 1-((2,6-difluorobenzyl)oxy)propan-2-ol. Conclusively, MRC-04 was shown to undergo fast degradation in aerobic conditions, especially when flooded, but not in an anaerobic condition in soil.
Keywords:
MRC-04, soil metabolism, aerobic condition, aerobic flooded condition, anaerobic conditionIntroduction
MRC-04 (5-(((2,6-difluorobenzyl)oxy)methyl)-5-methyl-3-(o-tolyl)-4,5-dihydroisoxazole), which is an analogue of a new turf herbicide methiozolin, and an excellent rice herbicide candidate. In soil applied MRC-04, it completely controlled susceptible and ALS-resistant barnyardgrass (Echinochloa oryzoides) at three-leaf stage (LS) at 300 g ai/ha and good safety to transplanted rice (Oryza sativa) at 600 g ai/ha in greenhouse. Tank-mixed MRC-04 (300 g ai/ ha) with several ALS-inhibiting rice herbicides with each standard application rate showed excellent herbicidal activity to the susceptible and ALS-resistant barnyardgrass at 2.5 LS without rice phytotoxicity in greenhouse and a paddy field. In the paddy field, in soil applied MRC-04, it demonstrated potent herbicidal efficacy to barnyardgrass applied at 0 days after transplanting (DAT) at 150 g ai/ha without rice phytotoxicity at 600 g ai/ha, and when applied at early- to middle-post rice growth stage (10 and 15 DAT), it showed excellent efficacy to barnyardgrass at 300 g ai/ha with acceptable rice phytotoxicity at 900 g ai/ha (Hwang et al., 2014).
Methiozolin is an isoxazolin compound as a parent molecule of MRC-04 and commercialized by Moghu Reserch Center Ltd. in Korean in 2010 and in Japan in 2016 as a turf herbicide controlling Poa species, major problematic weeds in golf courses (Lee et al., 2007; McCullough et al., 2013: Flessner et al., 2013; Koo et al., 2014; Koo et al., 2010). The environmental fate studies for methiozolin using radiolabeled compounds were investigated in soil under aerobic and anaerobic flooded conditions. Hwang et al. (2013) reported that methiozolin was metabolized steadily with time and the half-life was calculated 49 days, and the carbon dioxide and the non-extractable radioactivity increased as the soil aged to reach up to approximately 39 and 38% of the applied radioactivity until 120 days, respectively, but no volatile compound and major metabolite (>10% of the applied) was produced in the soil under the aerobic condition. The non-extractable residue was associated more with humin and fulvic fractions in the same condition. However, methiozolin degradation was limited in the soil under the anaerobic flooded condition (Hwang et al., 2013).
Although herbicidal activity of MRC-04 showed potent and residual efficacy against barnyardgrass in a paddy field, its metabolism information was not available. Therefore, in this paper we report on the metabolism of MRC-04 using radiolabeled compound in soil under aerobic, aerobic flooded, and anaerobic conditions, describing the mass balance, the degradation pattern, and the formation of metabolites.
Materials and Methods
Chemicals
The radiolabeled test compound, [benzyl-14C]MRC-04 (Figure 1), was synthesized at Korea Radiochemicals Center (Suwon, Korea). The radiochemical purity was >99%, and specific activity was 6.87MBq/mg. The compound was used without further purification. HPLC-grade acetonitrile, ethyl acetate, methanol and water were purchased from Duksan Co. (Ansan, Korea). All the other reagents and common chemicals were analytical grades and commercially available.
Test soil
A sandy loam soil was sampled from top 10 cm of a drained upland field (402, Hadae-ri, Gyeryong-myeon, Gongju-si, Chungcheongnam-do, Korea). The soil was air-dried and sieved at room temperature for 24 h then was stored at -20°C to maintain microbial activity prior to use (NIAST, 1988a; SSSA, 1996; SSSA, 1986; Rueppel et al., 1997). Soil characterization results were shown in Table 1.
Radioassay
Radioactivity of all liquid samples was measured by a liquid scintillation counter (LSC; Tri-Carb 2900TR, PerkinElmer, USA). Radioactivity in gross amounts of less than twice the background was considered to be the limit of determination accuracy. Ultima Gold® scintillation cocktail (4 mL) was used for aquatic and organic samples and Insta-Gel Plus® (4mL) was used for CO2 trapping. The non-extractable soil residue (0.2 g) was combusted by the sample oxidizer (Model 307, PerkinElmer, USA). The [14C]carbon dioxide produced was absorbed in Carbo-Sorb E® (5 mL) and mixed with PermaFluor E+® ( 1 0mL) scintillator for LSC counting. The efficiency of the oxidizer was determined using aliquots of Spec-Chec-14C check source for the automatic sample oxidizers, and was greater than 95%. Measurement of radio-activity was corrected by the oxidizer efficiency.
Chromatography
Identification of MRC-04 and its metabolites, and determination of radioactivity were performed using a PerkinElmer series 200 HPLC system (PerkinElmer, USA) equipped with an UV/vis detector and flow scintillation analyzer (Radiomatic 610TR, PerkinElmer, USA) by cochromatography with the authentic compounds. A reverse phase C18 column (Luna 5 μ PFP(2) 100A, 250 × 4.6 mm i.d., Phenomenex, USA) was used. The detection wavelength was 254 nm. The elution solvents were acetonitrile and water (containing 0.1% trifluoroacetic acid). A linear gradient (from 30% acetonitrile to 90% for 27 min) using a flow rate of 1.0 ml/min was adopted to separate the peaks of MRC-04 and the metabolites produced. The flow rate of the scintillation cocktail (Ultima-Flo M®) was 3 mL/min.
Extraction Efficiency
Soil samples (50 g, air-dry weight) were added with 5 mL of distilled water to adjust to 60% the field moisture holding capacity for the aerobic and anaerobic conditions, or 70 mL to form 2 cm water depth on the soil surface for the aerobic flooded condition. The [benzyl-14C]MRC-04 stock solution was prepared in 400 mg/L in acetonitrile, then 150 μL of the stock solution containing 60 μg of [benzyl-14C]MRC-04 was added to the soil sample. MRC-04 concentration in the soil (1.2 mg/kg) was equivalent to 0.3 kg/ha. Following the treatment, the soil was thoroughly mixed. After 30 min, the samples were extracted three times sequentially with acetonitrile/water (9:1, v/v, 100 mL), acetonitrile/water (1:1, v/v, 100 mL), and methanol/water/36% hydrochloric acid (9:1:0.1, v/v/v, 100 mL). Triplicate aliquots (0.5mL) of the soil extracts were analyzed by LSC to measure the radioactivity.
Soil Incubation
For the aerobic condition, the soil (50 g, air-dry weight) was weighed into the 100 mL incubation flask and added with 5 mL of the sterilized water. For the aerobic flooded condition, the soil (70 g, air-dry weight) in the incubation flask was flooded with 70 mL of the sterilized water to form >2 cm water depth from the soil surface (OECD, 2002; EPA, 2008a; EPA, 2008b; Chang et al., 2007). In case of the anaerobic condition, the soil (50 g, air-dry weight) was weighed into the 100 mL incubation flask and added with 5 mL of the sterilized water and maintained at the aerobic condition for 30 days, then the samples were added with 40 mL of N2 gas-purged sterilized water and maintained at the anaerobic condition by flowing a N2 gas.
All the soil samples were maintained in a flow-through metabolism chamber, and were pre-incubated at 25 ± 1°C for 2 weeks in the dark prior to the treatment. Each gas (air for aerobic and the aerobic flooded conditions; N2 for the anaerobic condition) was passed through the system at a flow rate of 20 mL/min through a 1.0 N sodium hydroxide solution to remove carbon dioxide, followed by distilled water to humidify. After pre-incubation, an aliquot of the application for [benzyl-14C]MRC-04 was applied to the soil for the aerobic and anaerobic conditions and onto the water surface for the aerobic flooded condition at a rate of 1.2 mg/kg as describe above. The soil samples treated were then again incubated in the flow-through system at 25 ± 1°C. The effluent gas from the chamber was passed through two ethylene glycol traps (40 mL) and two sodium hydroxide solution traps (1.0 N, 40 mL) in sequence to trap volatile compounds and [14C]carbon dioxide, respectively. The treated soil was sampled at 0, 3, 7, 14, 30, 60, 90 and 120 days for the soil under the aerobic condition, at 0, 3, 5, 14, 30 and 60 days for the soil under the aerobic flooded condition and 30, 0, 7, 14, 30, 60, 90 and 120 days for the soil under the anaerobic condition. Readjustment of the soil moisture content and sampling of [14C]carbon dioxide and volatile products were carried out every two weeks. Triplicate aliquots (1m L) of ethylene glycol and sodium hydroxide solution from the traps were counted by LSC for total radioactivity.
Extraction and Analysis of Soil
At each soil sampling date, three flasks per treatment were taken, and the soil samples (including water) were extracted with acetonitrile/water (9:1, v/v, 100 mL) by sonication (15 min) and shaking (30 min). The extract was centrifuged at 5000 rpm for 15 min, and then the supernatant was taken. The remaining soil was then sequentially extracted with 100 mL of acetonitrile/water (1:1, v/v) and 100 mL of methanol/water/36% hydrochloric acid (9:1:0.1, v/v/v). In each procedure, the same extraction was repeated two times, and analysis was done by LSC. Each extract from the soil was combined, acetonitrile of the pooled extract was removed under a reduced pressure (35°C), 0.05 mL of 36% hydrochloric acid solution was added to the aqueous solution, and the remainder was extracted twice with 100 mL of ethyl acetate. The ethyl acetate solution was concentrated in vacuo at 35°C and redissolved in 2 mL of acetonitrile. An aliquot of the solution was analyzed by Radio-HPLC to determine the concentration of the parent and its metabolites. All the post-extracted soil samples were air-dried, homogenized and weighed. Triplicate portions (0.2 g) were combusted with the oxidizer before LSC analysis.
Distribution of Non-extractable Radioactivity in Soil
The non-extractable residue by the extraction solvent system was fractionated with a strong base and acid into three fractions of humin, humic acid, and fulvic acid (Kim et al., 1998; Parsons, 1988). Ten grams (dry weight equivalent) of the non-extractable soil residue were extracted with 30 mL of 0.5 N sodium hydroxide solution by shaking for 24 h at room temperature. The extract was separated by centrifugation at 5000 rpm for 15 min and the supernatant (fulvic and humic acid) was collected. This procedure was repeated until the radioactivity of the extract reached the background level, and the extract were combined. Triplicate subsamples (0.2 g) of the precipitate (humin fraction) were combusted to determine the radioactivity content. The combined supernatant was added with concentrated hydrochloric acid to adjust to pH 1, and maintained at ambient temperature for 24 h. The mixture was then centrifuged at 5000 rpm for 15 min, and the supernatant (fulvic acid fraction) was collected. The resulting precipitate (humic acid fraction) was washed with 10 mL of 1 N hydrochloric acid solution and centrifuged (15 min, 5000 rpm). The supernatants were combined with the fulvic acid fraction. The volume of the combined solution was measured and 0.5 ml of the solution was analyzed by LSC. The humic acid precipitate was redissolved in 20 mL of 0.5 N sodium hydroxide solution, and the volume of the mixture was measured and then triplicate aliquots (0.1m L) were analyzed by LSC.
Isolation and Identification of Metabolites
Isolation and identification of the metabolite produced from MRC-04 were conducted from the soil under the aerobic flooded, because the major metabolites were produced only in that soil condition. To obtain a large amount of metabolites, 2 grams of MRC-04 was treated into the 1.5 kg soil under the aerobic flooded condition. The soil was extracted using acetonitrile/water (9:1, v/v) and acetonitrile/water (1:1, v/v) solution at 170 days. The solvent was evaporated and then the sample was extracted with ethyl acetate twice (80 mL, 70 mL). The extract was evaporated until complete dryness and redissolved with 2 mL acetonitrile, and the solution was analyzed by radio-HPLC to determine the metabolites. The extracted metabolites were isolated using precoated silica gel 60 F254 chromatoplate (20 × 20 cm, 0.5 mm layer thickness, Merck, Germany). The solvent system was n-hexane/ethyl acetate (6:1, v/v) and development was conducted twice. Finally, three purified metabolites were identified by nuclear magnetic resonance (NMR) spectrometer (Bruker, Germany) operating at 400 MHz for 1H and 13C with deuterochloroform (99.8%, Merck) and tetradeuteromethanol (99.9%, Cambridge Isotope Laboratories, Andover, MA, USA) as a solvent, respectively. EI positive spectra were recorded on High Resolution Mass Spectrometer (HRMS, JMS-700, JEOL, Japan).
Calculation of Half-Life
A pseudo-first-order kinetics model was used to calculate the half-life (NIAST, 1988b; Wang et al., 2009b; Dictor et al., 2008). The rates of degradation of [14C]MRC-04 were calculated by non-linear regression analysis using SigmaPlot 12.0 software (SPSS Science, USA)
Results and Discussion
Extraction Efficiency
[Benzyl-14C]MRC-04 was recovered from the test soil with high yield (92.3-100.8%) using acetonitrile/water (9:1, v/v) extraction solution. A small amount (<2.7%) was detected in the acetonitrile/water (1:1, v/v) extraction solution. Although most of radioactivity was recovered in the acetonitrile/water fraction, methanol/water/36% HCl (9:1:0.1, v/v/v) solution was used to extract metabolites, assuming they were potentially more polar or acidic than the parent compound (Hwang et al., 2013; Kim et al., 2003).
Mass Balance and Distribution. Aerobic condition
Mass balance was calculated at every sampling date by determining the radioactivity recovered from the solvent extracts, [14C]carbon dioxide, volatile compounds, and non-extractable residues (Wang et al., 2009a). The average mass balance ranged 90.2-97.8% of the applied during the study (Figure 2, A). The extractable radioactivity decreased with time and accounted for 30.2% of the applied at 120 days. The rate of degradation was rapid until first 60 days and then slower up to 120 days. The non-extractable residue increased with time and reached up to 35.9% of the applied at 120 days. The increasing rate was fast until 60 days and then slower up to 120 days. [14C]Carbon dioxide increased steadily as the soil aged and accounted for 24.1% of the applied at 120 days. No volatile products from the soil were detected throughout the study. From these results, we could assume that about 70% of MRC-04 treated was metabolized, 36% of those were rapidly bound to the soil, and 24% of those were further completely decomposed to carbon dioxide by aerobic microbes in the soil. The radioactivity of the non-extractable was determined 8.4, 15.1 and 12.5% of the applied in fulvic acid, humic acid, and humin fractions at 120 days, respectively, indicating bound residue was associated relatively more with humic acid and humin (Figure 3, A).
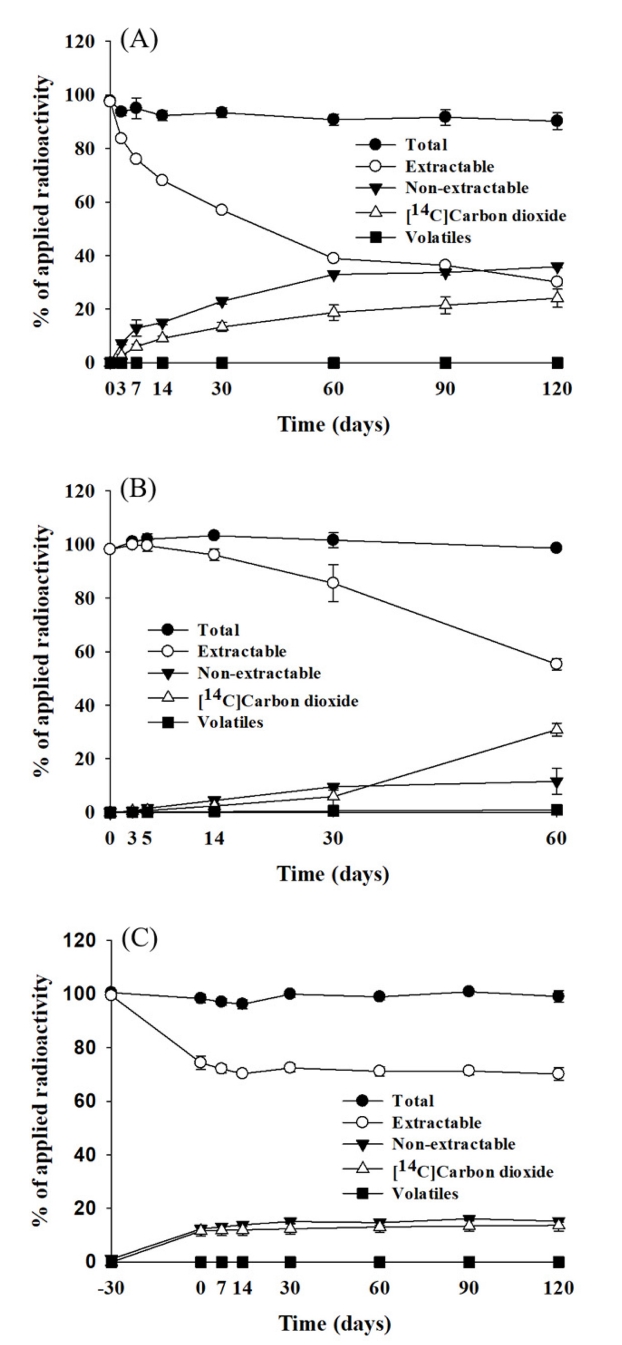
Distribution of radioactivity in the soil treated with [benzyl-14C]MRC-04 under the aerobic (A), aerobic flooded (B) and anaerobic (C) conditions. Each data point represents means± SD.
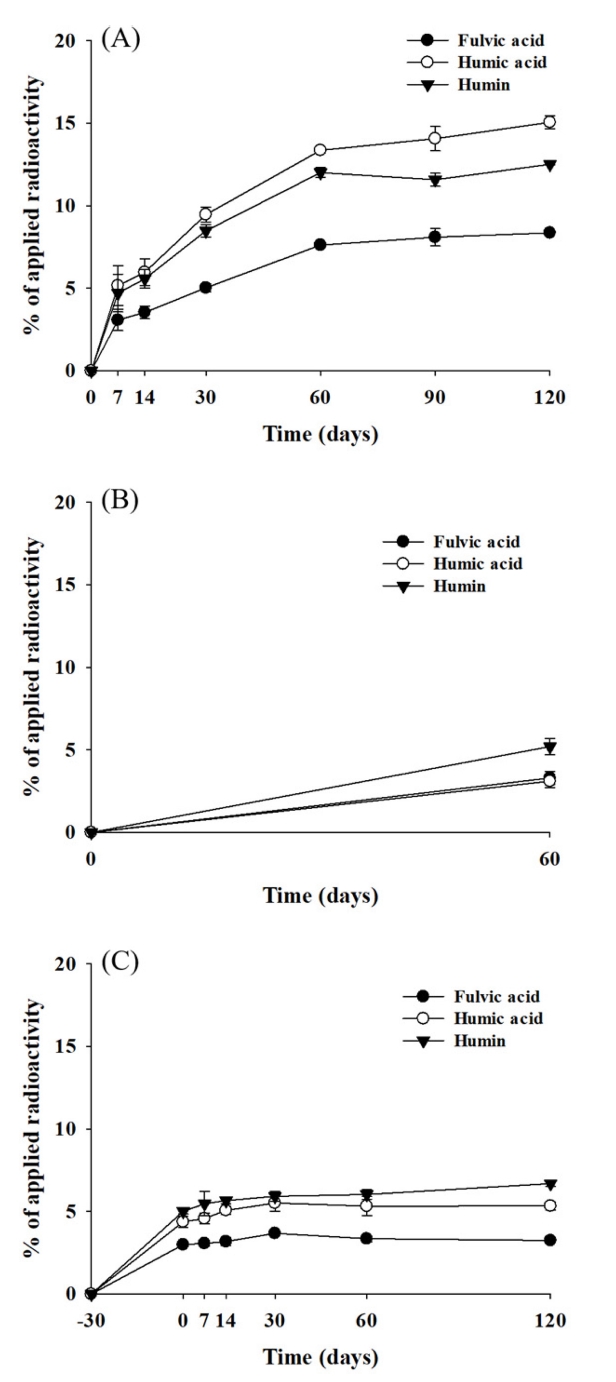
Distribution of non-extractable radioactivity in the soil treated with [benzyl-14C]MRC-04 under the aerobic (A), aerobic flooded (B) and anaerobic (C) conditions.
The average mass balance over the study was 98.1-103.3% of the applied radioactivity (Figure 2, B). The radioactivity of the extract from the soil slowly decreased to 85.5% of the applied until first 30 days, then more rapidly declined to 55.3% at 60 days. The non-extractable radioactivity steadily increased and accounted for 11.6% of the applied at 60 days. [14C]Carbon dioxide slowly increased to 5.9% of the applied for 30 days, but thereafter rapidly increased up to 30.9% at 60 days. The results indicate that MRC-04 and/or the metabolites produced until 30 days might be further rapidly decomposed completely after 30 days. A small amount of volatile compound was detected (<1.0%) during the study. The radioactivity of the non-extractable was distributed to 3.3, 3.1 and 5.2% of the applied in fulvic acid, humic acid, and humin fractions at 30 days, respectively, indicating bound residue was associated more with humin (Figure 3, B)
The mass balance ranged from 94.8 to 100.9% of the applied over the study (Figure 2, C). During the aerobic condition period for 30 days, the radioactivity of the solvent extract from the soil declined with time and accounted for 74.4% of the applied, non-extractable radioactivity and [14C]carbon dioxide increased to 12.4 and 11.6%, respectively, but no volatile compound was detected. However, after converted to the anaerobic condition, the solvent extractable radioactivity decreased slightly with time and accounted for 70.2% of the applied, the non-extractable radioactivity and [14C]carbon dioxide increased to 15.3 and 13.7%, respectively, and no volatile compound was detected until 120 days. A small amount of the treated radioactivity was bound to the soil (2.9%) and completely decomposed to CO2 (2.1%) during the anaerobic condition period. The results indicated a minimal metabolism activity of the soil microbes under the anaerobic condition. The radioactivity of the non-extractable was determined to be 3.2, 5.3 and 6.7% of the applied in fulvic acid, humic acid, and humin fractions at 120 days, respectively, indicating bound residue was associated relatively more with humic acid and humin (Figure 3, C).
Degradation of [14C]MRC-04 and Formation of Metabolites
Degradation of [benzyl-14C]MRC-04 and formation of metabolites in the soil under the aerobic, aerobic flooded, and anaerobic conditions were shown in Figure 4. In the soil under the aerobic condition, MRC-04 decreased to 32.1% of the applied until 60 days, after then and further to 23.6% at 120 days (Figure 4, A). The estimated half-life was 63.0 days. Four minor metabolites were produced and the each ranged 0.2-5.4% of the applied, and the combined percentages for all the metabolites at each sampling date were from 4.9 to 8.1%, and peaked at 14 days (Figure 4, A).
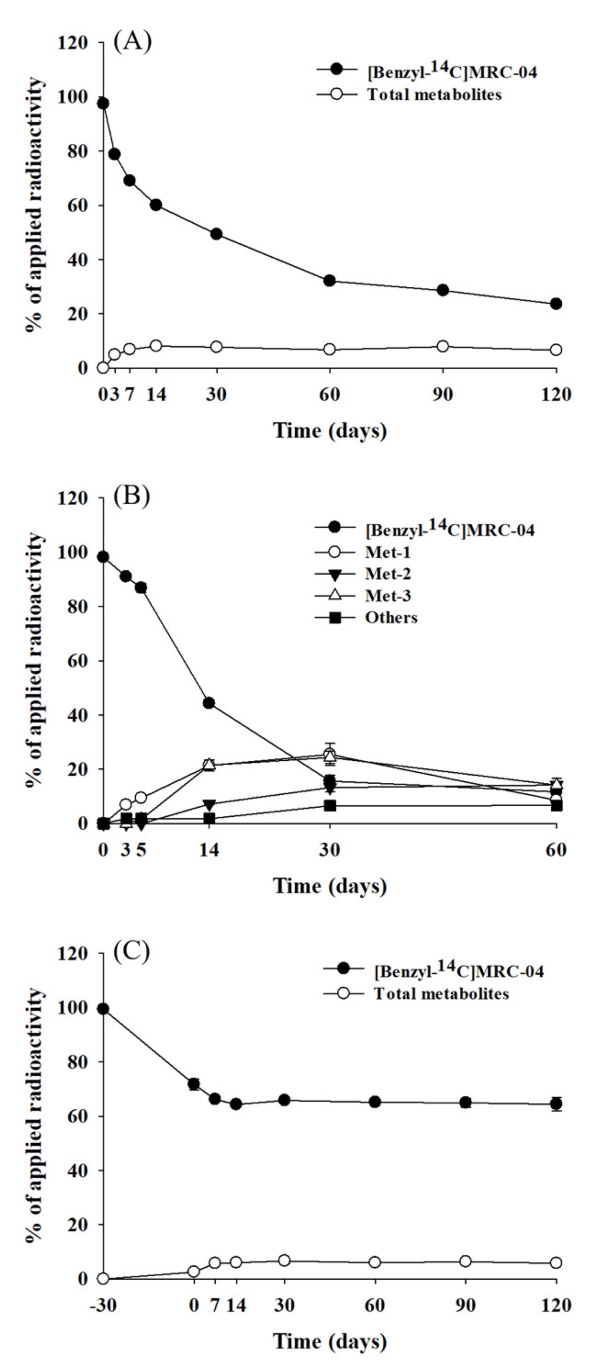
Degradation of [benzyl-14C]MRC-04 and formation of metabolites in the soil under the aerobic (A), aerobic flooded (B) and anaerobic (C) conditions.
In the soil under the aerobic flooded condition, MRC-04 was decreased slowly to 86.8% of the applied until 5 days, and rapidly to 11.7% at 60 days (Figure 4, B). The calculated half-life was 17.9 days. Three major metabolites were detected and identified as 2,6-difluorobenzoic acid (Met-1), 4-((2,6-difluorobenzyl)oxy)-3-hydroxy-3-methyl-1-(o-tolyl)butan-1-one (Met-2), and 1-((2,6-difluorobenzyl) oxy)propan-2-ol (Met-3) (Figure 4, B). Met-1 and Met-3 increased with time and peaked up to 25.5 and 24.4% of the applied at 30 days, thereafter decreased to 8.6 and 14.2% at 60 days, respectively. On the other hand, Met-2 increased steadily and accounted for 14.2% of the applied at 60 days. Beside the major metabolites, 6 minor metabolites were detected, and the amount of each metabolite was 0.3-2.5% of the applied. The combined minor metabolites ranged from 1.8 to 6.7% of the applied and were maximum detected at 60 days.
In the soil under the anaerobic condition, MRC-04 decreased rapidly and accounted for 71.8% for first 30 days under the aerobic condition; however, after converted to anaerobic condition only 7.4% of the applied was additionally degraded until 120 days (Figure 4, C). Therefore, the half-life in the anaerobic condition could not be calculated. Five minor metabolites were detected and the amount of each ranged from 0.2 to 2.5% of the applied. Maximum combined percentage for all minor metabolites was 6.7% of the applied at 60 days (Figure 4, C). Each representative chromatograms are shown in Figure 5.
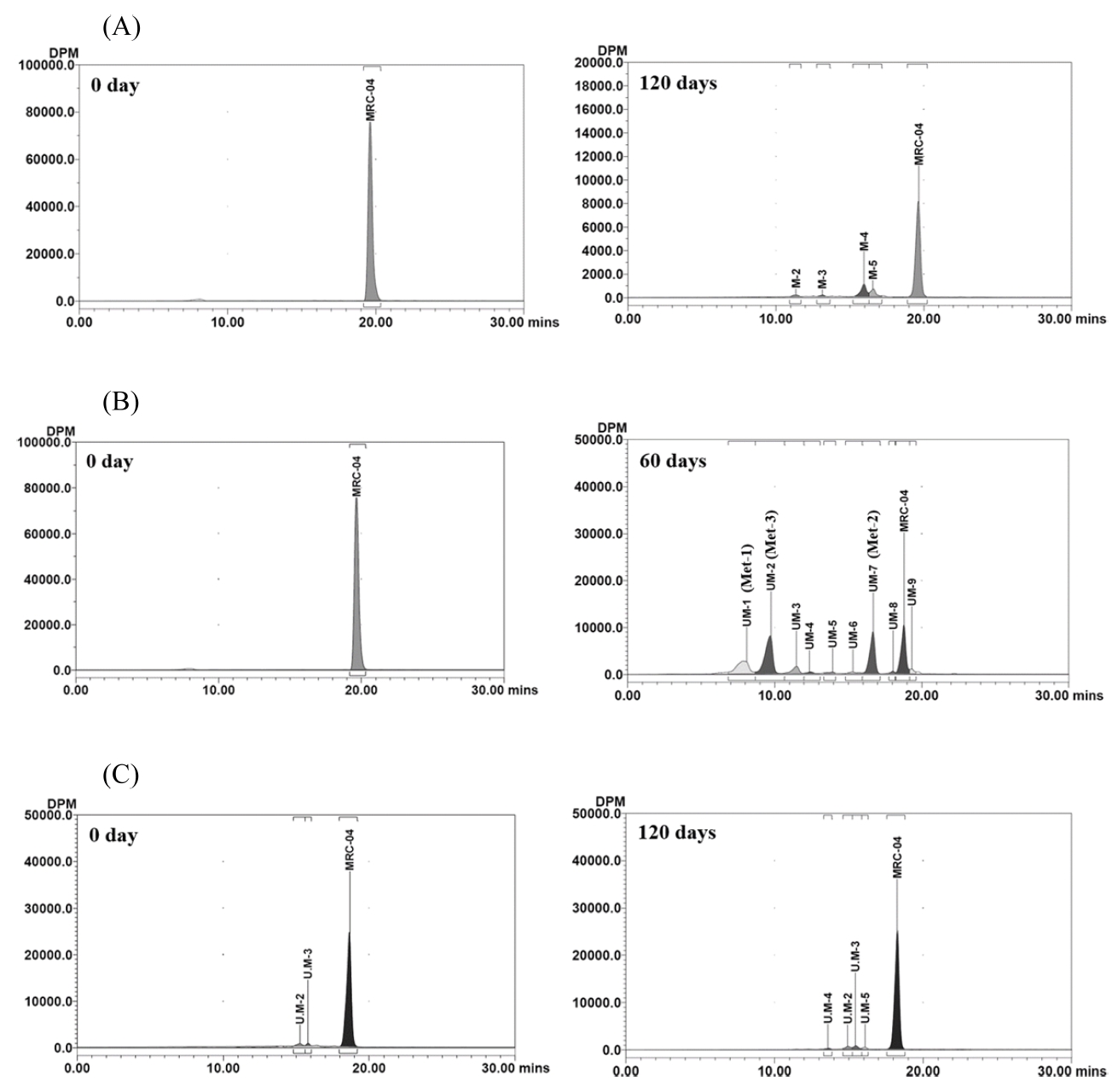
Representative radio-HPLC chromatograms of solvent extracts from the soil under the aerobic (A), aerobic flooded (B) and anaerobic (C) conditions after application of [benzyl-14C]MRC-04.
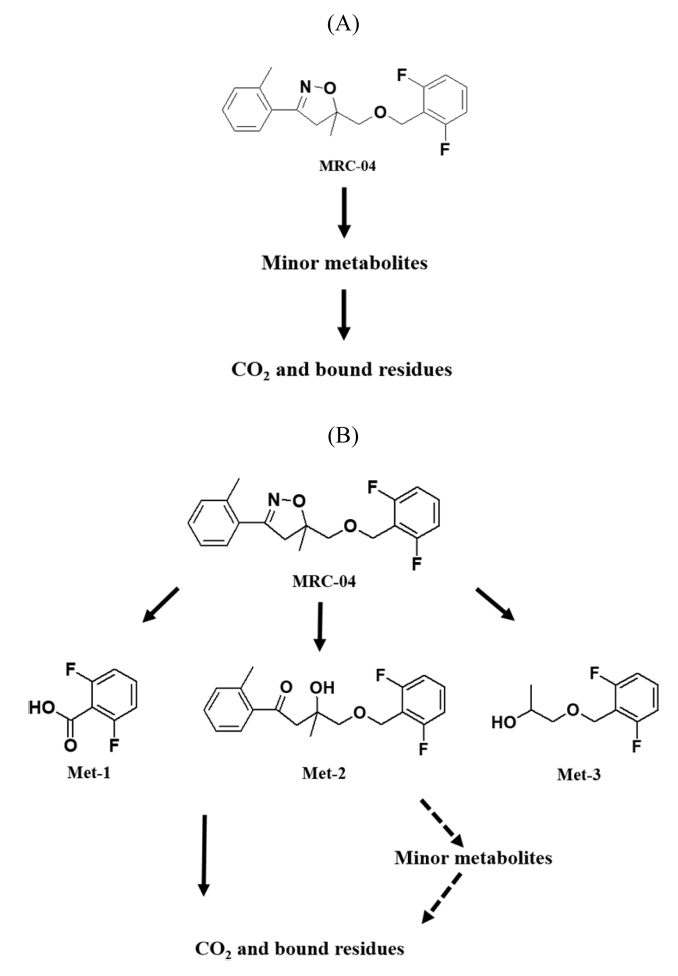
In summary, MRC-04 was rapidly degraded in the aerobic and aerobic flooded conditions, but not in the anaerobic condition. The major metabolites were detected only in the aerobic flooded condition. These results first suggested that MRC-04 metabolism in soil is mainly associated with aerobic microbes, when the aerobic and aerobic flooded condition were compound, CO2 was produced more in the flooded condition, while more soil bound residue was measured in the non-flooded condition. These results indicate that the larger portion of metabolites produced from MRC-04 were further metabolized and bound to soil under the non-flooded condition, while the metabolites were completely decomposed to carbon dioxide rather than binding to soil under the aerobic flooded condition.
Identification of Isolated Metabolites
The NMR data and chemical structure of the identified metabolites, Met-1, Met-2 and Met-3, are shown in Table 3 and Figure 6, respectively.
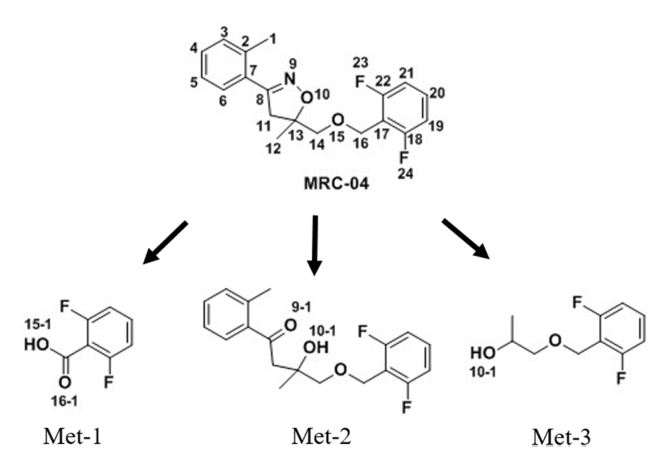
Chemical structure of MRC-04 and the identified metabolites from the soil under the aerobic flooded condition.
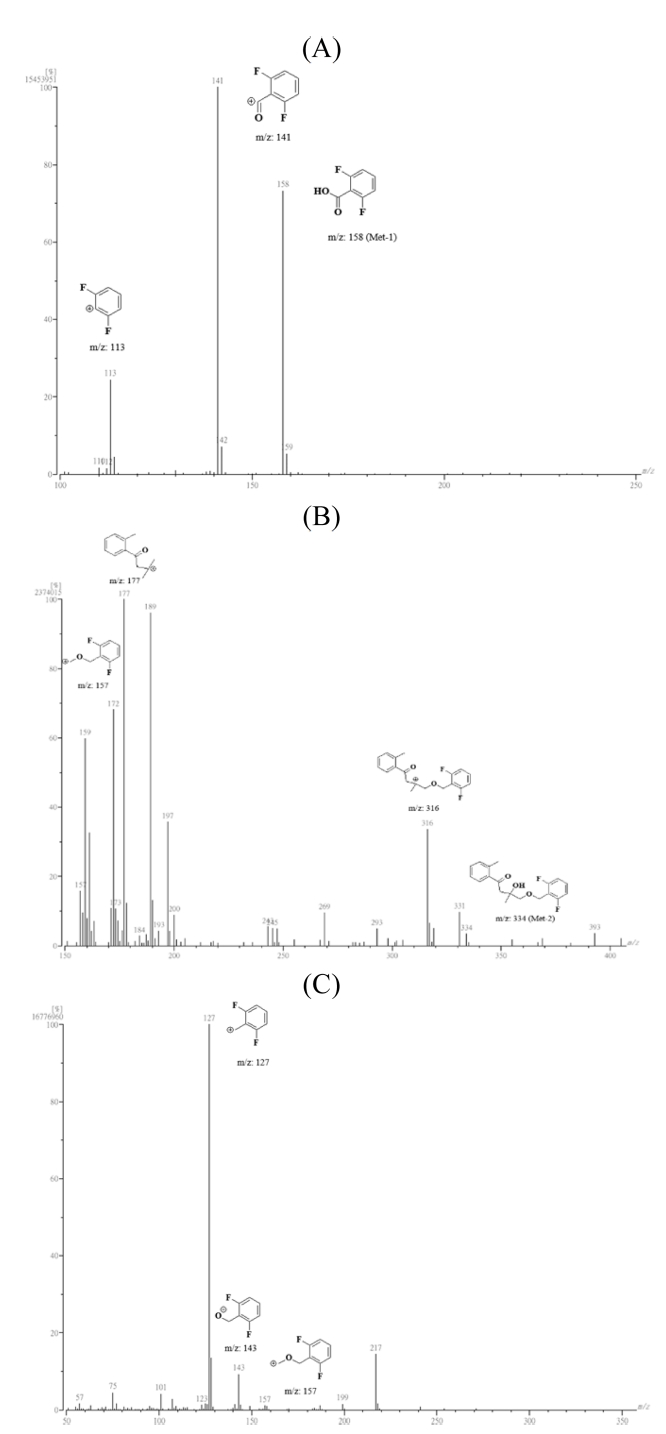
In the 1H-NMR spectrum, three proton signals at δ 7.06 (1H), 7.52 (1H) and δ 7.06 (1H) at C19, 20 and 21 were indicated the presence of the difluorobenzene ring, respectively, but a hydroxyl proton peak of 15-1 or 16-1 was not detected. The signals in the 13C-NMR spectrum for Met-1 were assigned as follows: δ 163.13 (C16), 132.52 (C17), 161.44 (C18), 132.52 (C19), 158.92 (C20), 132.52 (C21), and 161.51 (C22). The signal of C16 was significantly shifted to the down field, and the signals in the benzene ring were slightly moved to the down field compared with those of MRC-04. The EI-MS spectrum showed a molecular mass in peak at m/z 158 and a fragment peak at m/z 141 and 113 (Figure 7, A). Met-1 was observed m/z 158.0175 by HRMS. On the basis of these results, Met-1 was identified as 2,6-difluorobenzoic acid (m/z 158.0179), and might be produced as the cleavage of ether bond between isoxazoline ring and difluorobenzene ring of MRC-04.
In the 1H-NMR spectrum, three proton signals at δ 6.88 (1H), 7.39, and δ 6.88 (1H) at C19, 20, and 21 were indicated the presence of difluorobenzene ring, respectively. Four proton signals at δ 7.27 (1H), 7.27 (1H), 7.27 (1H), and 7.64 (1H) at C3~C6, and 3 proton signal at δ 2.47 at C1 were shown to the presence of toluene ring. Four proton signals δ 3.49 (2H) and 4.64 (2H) at C14 and C16 were assigned to the ether bond, respectively. Two proton signals at 3.32 (1H) and 2.94 (1H) at C11 and 3 proton signals at 1.31 (3H) at C12 were indicated the presence of carbons of isoxazoline ring, however 1 hydroxyl proton at 10-1 was detected and the signals at C11 and C12 slightly moved to the down field affected by oxygen at 9-1 and 10-1. The signal in the 13C-NMR spectrum for Met-2 were assigned as follows: δ 21.13 (C1), 138.00 (C2), 131.59 (C3), 131.90 (C4), 125.68 (C5), 128.80 (C6), 138.33 (C7), 163. 14 (C8), 47.28 (C11), 25.28 (C12), 72.03 (C13), 76.77 (C14), 60.21 (C16), 111.30 (C17), 160.65 (C18), 111.30 (C19), 130.19 (C20), 111.30 (C21), and 160.73 (C22). The signals of C7 and C8 were slightly shifted to the down field, but that of C14 was moved to the up field slightly compared to those of MRC-04. The EI-MS gave a molecular mass ion peak at m/z 334 and its fragment peaks (m/z 157, 177, and 316) (Figure 7, B). Met-2 was observed m/z 334.1376 by HRMS. On the basis of the results, Met-2 was identified as 4-((2,6-difluorobenzyl)oxy)-3-hydroxy-3-methyl-1-(o-tolyl) butan-1-one (m/z 334.1381), and it might be produced by reduction of N-O bond in isoxazoline ring followed by hydrolysis of exchanging nitrogen to oxygen (Kalgutkar et al., 2003; Tschirret and Wood, 2003).
In the 1H-NMR spectrum, 3 proton signals at δ 6.94 (1H), 7.31 (1H), and 6.94 (1H) at C19, C20, and C21, respectively, showed the presence of the difluorobenzene ring. Four proton signals at δ 3.40 (2H) and 4.67 (2H) at C14 and C16, respectively, were assigned to the ether bond. Three proton signal at δ 1.15 at C12 was detected, and 1 proton signal was at δ 3.98 at C13. The signal in the 13C-NMR spectrum for Met-3 were assigned as follows: δ 18.47 (C12), 66.33 (C13), 75.89 (C14), 60.33 (C16), 111.27 (C17), 160.67 (C18), 111.27 (C19), 130.33 (C20), 111.27 (C21), and 160.75 (C22). The signals of C12 and C13 were slightly shifted to the up field compared with those of MRC-04. The EI-MS spectrum did not showed a molecular ion peak, but its fragment peaks (m/z 127, 143, and 157) (Figure 7, C). On the basis of the results, Met-3 was identified as 1-((2,6-difluorobenzyl)oxy)propan-2-ol, and it might be produced by dissociation of N-O bond and carbon cleavage between C11 and C13 in the isoxazoline ring.
Conclusion
In the present study, mass balances from soil experiments under aerobic (90.2 - 97.8), aerobic flooded (98.1 - 103.3%) and anaerobic flooded conditions (94.8 - 100.9%) for [benzyl-14C]MRC-04 provided the reliability of results obtained in this study. The half-life of MRC-04 was calculated to be approximately 63.0 days in soil under aerobic condition, when MRC-04 was treated at a concentration of 0.3 kg/ha on a sandy loam soil at 25 ± 1°C. And half-life of MRC-04 was calculated to be approximately 17.9 days in soil under aerobic flooded condition. However, a small amount of [14C]MRC-04 degraded in soil under anaerobic flooded conditions. Three major metabolites were detected in the soil under aerobic flooded condition and identified as 2,6-difluorobenzoic acid, 4-((2,6-difluorobenzyl)oxy)-3-hydroxy-3-methyl-1-(o-tolyl) butan-1-one and 1-((2,6-difluorobenzyl)oxy)propan-2-ol. Conclusively, MRC-04 was shown to undergo fast degradation in aerobic conditions, especially when flooded, but not in an anaerobic condition in soil. The proposed metabolic pathway of MRC-04 in soil under aerobic, aerobic flooded and anaerobic conditions was depicted in Figure 8.
Literature cited
-
Chang HR, Koo SJ, Kim K, Ro HM, Moon JK, et al., 2007. Soil metabolism of a new herbicide, [14C]pyribenzoxim, under flooded conditions. J. Agric. Food Chem. 55(15):6206-6212.
[https://doi.org/10.1021/jf070760u]

-
Dictor MC, Baran N, Gautier A, Mouvet H, 2008. Acetochlor mineralization and fate of its two major metabolites in two soils under laboratory conditions. Chemosphere. 71(4):663-670.
[https://doi.org/10.1016/j.chemosphere.2007.10.066]

- EPA, 2008a. Fate, Transport and Transformation Test Guidelines. OPPTS 835.4100, Aerobic Soil Metabolism.
- EPA, 2008b. Fate, Transport and Transformation Test Guidelines. OPPTS 835.4200, Anaerobic Soil Metabolism.
-
Flessner ML, Wehtje GR, McElroy JS, 2013. Methiozolin absorption and translocation in annual bluegrass (poa annua). Weed Sci. 61(2):201-208.
[https://doi.org/10.1614/WS-D-12-00128.1]

- Hwang KH, Cho NG, Jeon MS, Lee DG, Kim SH, et al., 2014. Herbicidal efficacy of MRC-04 under paddy conditions. 13th IUPAC Conf. 225.
-
Hwang KH, Lim JS, Kim SH, Chang HR, Kim K, et al., 2013. Soil metabolism of [14C]Methiozolin under aerobic and anaerobic flooded conditions. J. Agric. Food Chem. 61(28):6799-6805.
[https://doi.org/10.1021/jf400199u]

-
Kalgutkar AS, Nguyen HT, Vaz AND, Doan A, Dalvie DK, et al., 2003. In vitro metabolism studies in the isoxazole ring scission in the anti-inflammatory agent leflunomide to its active α-cyanoenol metabolite A771726: Mechanistic similarities with the cytochrome P450-catalyzed dehydration of aldoximes. Drug Metabolism and Disposition. 31(10):1240-1250.
[https://doi.org/10.1124/dmd.31.10.1240]

-
Kim JH, Kang KG, Park CK, Kim K, Kang BH, et al., 1998. Aerobic soil metabolism of flupyrazofos. Pestic. Sci. 54:237-243.
[https://doi.org/10.1002/(SICI)1096-9063(1998110)54:3<237::AID-PS817>3.0.CO;2-B]

-
Kim JH, Lee YS, Keum YS, Kim MK, Kang SH, et al., 2003. Metabolism of ethaboxam in soil. In Environmental fate and effects of pesticides, ACS symposium series, 853: 65-78.
[https://doi.org/10.1021/bk-2003-0853.ch004]

-
Koo SJ, Hwang KH, Jeon MS, Kim SH, Lim JS, et al., 2010. Development of the new turf herbicide methiozolin. Kor. J. Weed Sci. 30(4):323-329.
[https://doi.org/10.5660/KJWS.2010.30.4.323]

-
Koo SJ, Hwang KH, Jeon MS, Kim SH, Lim JS, et al., 2014. Methiozolin [5-(2, 6-difluorobenzyl)oxyethyl-5-methyl-3,3(3-methylthiophen-2-yl)-1, 2-isoxazoline], a new annual bluegrass (Poa annua L.) herbicide for turfgrasses. Pest Manag Sci. 70:156-162.
[https://doi.org/10.1002/ps.3541]

- Lee JN, Koo SJ, Hwang KH, Hwang IT, Jeon DJ, et al., 2007. Mode of action of a new isoxazoline compound. Proc. 21st APWSS Conf. 591-601.
-
McCullough PE, de Barreda DG, Yu J, 2013. Selectivity of methiozolin for annual bluegrass (Poa annua) control in creeping bentgrass as influenced by temperature and application timing. Weed Sci. 61(2):209-216.
[https://doi.org/10.1614/WS-D-12-00135.1]

- National Institute of Agricultural Science and Technology, 1988a. Methods of Soil Chemistry Analysis. Suwon, Korea.
- National Institute of Agricultural Science and Technology, 1988b. Soil, plant, soil microbiology. Suwon, Korea.
- OECD, 2002. OECD guidelines for the testing of chemicals. Test No. 307, Aerobic and anaerobic transformation in soil (Adopted 24 April 2002).
- Parsons JW, 1988. Isolation of humic substance from soils and sediments. In humic substances and their Role in the Environment; Frimmel, F. H.; Christman, R.F., Eds.; Wiley: New York, pp 3-14.
-
Rueppel ML, Brightwell BB, Schaefer J, Marvel JT, 1997. Metabolism and degradation of glyphosate in soil and water. J. Agric. Food Chem. 25(3):517-528.
[https://doi.org/10.1021/jf60211a018]

- Soil Science Society of America, 1986. Methods of Soil Analysis, Part I, Physical and Mineralogical methods. Madison, WI, USA.
- Soil Science Society of America, 1996. Methods of Soil Analysis, Part 3, Chemical Methods. Madison, WI, USA.
-
Tschirret RA, Wood HB, 2003. Substituent effect on the reductive N-dearylation of 3-(indol-1-yl)-1, 2-benzisoxazoles by rat liver microsomes. Drug Metabolism and Disposition. 31(8):999-1004.
[https://doi.org/10.1124/dmd.31.8.999]

-
Wang H, Ye Q, Yue L, Han A, Yu Z, et al., 2009a. Fate characterization of a novel herbicide ZJ0273 in aerobic soils using multi-position 14C labeling. Science of the Total Environment, 407(13):4134-4139.
[https://doi.org/10.1016/j.scitotenv.2009.03.028]

-
Wang H, Ye Q, Yue L, Yu Z, Han A, et al., 2009b. Kinetics of extractable residue, bound residue and mineralization of a novel herbicide, ZJ0273, in aerobic soils. Chemosphere. 76(8):1036-1040.
[https://doi.org/10.1016/j.chemosphere.2009.04.051]

Jong-Soo Lim, Moghu Research Center Ltd., Ph.D. student, https://orcid.org/0000-0002-8843-6807
Ki-Hwan Hwang, Moghu Research Center Ltd., Doctor of Philosophy, https://orcid.org/0000-0002-6887-3639
Sung-Hun Kim, Moghu Research Center Ltd., Ph.D. student, https://orcid.org/0000-0001-5992-7458
Jun-Ho Nam, Moghu Research Center Ltd., Master, https://orcid.org/0000-0003-1409-7344
Suk-Jin Koo, Moghu Research Center Ltd., Doctor of Philosophy, https://orcid.org/0000-0002-2908-758X
“Methodology, Lim J.S. and Hwang K.H.; Validation, Lim J.S., Hwang K.H. and Kim S.H.; Investigation, Lim J.S., Kim S.H. and Nam J.H.; Data Curation, Lim J.S. and Hwang K.H.; Writing – Original Draft Preparation, Lim J.S.; Writing- Review & Editing, Hwang K.H. and Koo S.J.; Supervision, Koo S.J.”