Disruption of Juvenile Hormone Receptor Binding in Tobacco Cutworm Larvae by Gladiolus gandavensis extract
Abstract
Tobacco cutworm (Spodoptera litura [Fabricius]) is a destructive insect pest distributed in densely cultivated parts of Asia and Australia. This insect shows an explosive increase in its population in summer. Furthermore, tobacco cutworm larvae are very difficult to control because of their strong resistance to commonly applied chemical insecticides. To identify alternative pesticides that circumvent toxic effects to the environment and the threat of insecticide resistance, we aimed to examine plant extracts with insect growth regulatory activity targeting juvenile hormones (JHs). Using RNA sequencing and de novo transcriptome assembly, S. litura cDNA sequences of the JH receptor methoprene-tolerant (Met) and steroid receptor coactivator (SRC) were determined and used to generate yeast two-hybrid bait and prey plasmids. The JH/JH analog-mediated binding of Met and SRC was measured by assaying β-galactosidase activity. In the plant extract activity assay, we tested 1,628 plant extracts in transformants bearing Met and SRC. We identified 67 plant extracts with high JH disruptor (JHD) activity in tobacco cutworm, of which Gladiolus gandavensis extract showed the highest JHD activity. Plant-based JHDs might serve as a viable and sustainable alternative to chemical pesticides to control tobacco cutworm infestations in the field.
Keywords:
insect growth regulator, Gladiolus gandavensis, pest control, plant extract, tobacco cutwormIntroduction
Tobacco cutworm (Spodoptera litura [Fabricius] [Lepidoptera: Noctuidae]) is an omnivorous insect pest distributed in Asia and Australia, and it causes huge economic losses by damaging nearly 120 types of important field crops, such as beans, cabbage, tomatoes, tobacco, and peppers (Malarvannan et al., 2010). In Korea, its occurrence has been reported to be centered in the southern regions, where agriculture has been predominant since 1990 with up to five crop cycles a year, providing ample host opportunities for this pest (Han et al., 2018; Holloway, 1989; Jung et al., 2010). Spodoptera litura is characterized by an explosive increase in its population density in a short period of time, and the growth of larvae is promoted in the summer season due to its high fertility and strong resistance to high temperatures (Bae and Park, 1999).
Effective control measures for tobacco cutworm are urgently warranted to reduce economic damage. However, tobacco cutworm larvae exhibit strong resistance and low susceptibility to most of the chemical insecticides commonly applied in the field. In particular, larvae of the third instar and later (Sayyed et al., 2014) are very difficult to control with chemicals. In addition, chemical pesticides are known to cause severe environmental toxicity (Nauen 2007; Rivero et al., 2010; Ahn et al., 2013). Accordingly, there is an increasing demand for pesticides that confer low pesticide resistance and are environmentally friendly. Owing to the need to reduce the use of chemical insecticides and introduce alternative and effective methods to control tobacco cutworm, various researchers are actively exploring the use of juvenile hormones (JHs), a class of insect growth regulators (IGRs), and their receptors. IGRs control the transformation and molting processes of insects and have low environmental toxicity (Lee et al., 2015; Palli and Retnakaran, 2001; Smith, 1995). For instance, in Drosophila melanogaster, methoprene-tolerant (Met; a JH receptor) was found to have homology to the basic helix-loop-helix (bHLH) Per-Arnt-Sim (PAS) transcription factor family (Ashok et al., 1998; Wilson and Ashok, 1998). The bHLH-PAS protein (also known as Taiman, FISC, or SRC) and the JH receptor bind to each other only in the presence of the JH when the bHLH-PAS domain is activated, thereby regulating the gene in a JH-dependent manner (Lee et al., 2015).
In this study, JH disruptor (JHD) activity of 1,628 plant extracts including gladiolus extracts was tested against tobacco cutworm by establishing a screening system based on a yeast two-hybrid system of Met and SRC genes in tobacco cutworm. And it can be used as an insecticide, Terpenes are the largest among plant secondary metabolites and have been extensively studied for their potential as antimicrobial, insecticidal, and weed control agents. The major terpene constituents of the essential oil of gladiolus were linalool (Kahriman N et al., 2012). As a pesticide, Linalool is intended for use indoors to control pests (fleas and ticks) on pets and the spaces they inhabit by affecting the insect’s nervous system (Ninkuu et al., 2021). Through this approach, we aimed to identify promising candidate substances that can control tobacco cutworm in an effective and environmentally friendly manner.
Materials and Methods
Insects
Tobacco cutworm larvae were obtained from the National Institute of Agricultural Sciences, Rural Development Administration (Wanju, JB, Korea), and continuously reared (Kim et al., 2008) at 25oC ± 2oC and 50–60% relative humidity in petri dishes (90 X 15, SPL; Pocheon, GG, Korea) under a 16L:8D photoperiod. The larvae were fed artificial feed (Goh et al., 1990).
Chemicals
JH III, pyriproxyfen, fenoxycarb, methoprene, hydroprene, and kinoprene used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), and JH I and II were purchased from MuseChem (Fairfield, NJ, USA). A total of 1,628 plant extracts including gladiolus extracted with methanol at a concentration of 20 mg/mL were obtained from the Plant Extract Bank of Korea (Daejeon, Korea) for use in our experiments. In addition, methyl linderone was used as a positive control, which was separated and stored as in a previous study (Lee et al., 2015).
RNA sequencing and de novo transcriptome assembly
Approximately 99 million (101 bases) reads were generated for S. litura using Illumina RNA sequencing, and de novo assembly was performed using Trinity (Grabherr et al., 2011). Met and Taiman of Drosophila melanogaster were registered in GenBank as query sequences using TBLASTN after confirming the encoding cDNA sequences of Met and SRC.
Tobacco cutworm JHD activity system
Full-length cDNA encoding the Met open reading frame (ORF) (i.e., M1-L527 [Genbank ID: MW759040]) and partial cDNA encoding the SRC ORF (i.e., M1-P577 [Genbank ID: MW759041]) for tobacco cutworm were introduced into yeast two-hybrid bait and prey plasmids, respectively. The bait plasmid was prepared by cloning Met cDNA into the GAL4 DNA-binding domain of the pGBKT7 vector (Clontech, Mountain View, CA, USA), and the prey plasmid was prepared by cloning the GAL4-AD fusion plasmid (Clontech) of the pGADT7 vector containing SRC.
S. litura Met forward: 5’CGTACATATGACATCTTCAGGTGGACCAAG3’
S. litura Met reverse: 5’CGTAGAATTCTTACAGATCTACTTCATTAAC3’
S. litura SRC forward: 5’CGTACATATGGTACAAACAGAACCTGTGC3’
S. litura SRC reverse: 5’CGTAGAATTCGGGCGTGTGCGGCGTGTG3’
Yeast β-galactosidase assay (JH/JHA assay)
Both Met bait and SRC prey plasmids were transformed into yeast Y187 cells, and the transformed Y187 cells were incubated at 30oC in SD-Leu/−Trp double dropout medium until the OD600 reached 0.3–0.4. The cells were then centrifuged and suspended in a double volume of culture media. The cells were further incubated for 3 h, and 100 μL of each was dispensed in a 96-well plate (OD600 = 0.2–0.3). Both JH and JH analog (JHA) at concentrations of 0, 0.01, 0.1, 1, and 10 ppm were applied to the cells and incubated for an additional 3 h. β-Galactosidase activity was analyzed using a β-galactosidase assay kit (Thermo Scientific, Waltham, MA, USA). In addition, OD420 was measured after the assay reaction mixtures were incubated at 25oC for 12 h.
Plant extract activity assay
The transformant comprising Met and SRC of tobacco cutworm was incubated in SD-Leu/−Trp double dropout medium at 30oC until the OD600 reached 0.3–0.4. The cells were then centrifuged and suspended in a double volume of culture media, followed by an additional 3 h incubation. After 0.1 ppm of juvenile hormone was dispensed into the wells of a 96-well plate containing 100 μL of the culture, 200 ppm of each of the 1,628 plant extracts including gladiolus extracts was applied. In addition, 10 ppm of methyl linderone was used as a positive control, and DMSO, which is a vehicle used to dissolve plant extracts, was used as a negative control. Each plate treated with plant extracts was further incubated at 30oC for 5 h. After the incubation, each plate was treated with a reagent from a β-galactosidase assay kit (Thermo Scientific), and β-galactosidase activity was compared and analyzed by measuring the OD420. Normalization was performed using the measured values. For normalization, the specific JHD activity of each plant extract was calculated using the following equation:
Bioassay
Artificial feed was prepared by adding red pepper leaf and 20 mg/mL of methanol extract of gladiolus at 10% of the weight of the artificial feed. Ten tobacco cutworm larvae at 1~3 instar stages were placed in each petri dish, and the development status of each was compared by measuring the weight by instar stage according to the date when supplying artificial feed + red pepper leaf extract 10% (w/w, negative control) and artificial feed + gladiolus extract 10% (w/w, treatment) every 2 days. A group receiving only artificial feed was used as a control group.
RNA extraction, primers, and RT-qPCR analysis
Total RNA was extracted using the RNeasy kit (Qiagen, Hilden, Germany) from first instar larvae fed with control, pepper leaf, and gladiolus extract. For RT-qPCR, cDNA was synthesized from 1 μg of total RNA using the iScript cDNA Synthesis kit (BIO-RAD, Hercules, CA, USA). After the concentration of each cDNA was measured using a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Middlesex County, MA, USA), the cDNA was diluted and used for qRT-PCR. Primers of target genes (Kr-h1, Hsp83) were designed using primer 3 (https://bioinfo.ut.ee/primer3-0.4.0/). After 20 μL reaction mixtures of the diluted cDNA were prepared using BIO-RAD and SsoAdvanced Universal SYBR Green Supermix (BIO-RAD), RT-qPCR was performed with the following conditions: 35 cycles of 94oC for 15 s, 60oC for 20 s, and 72oC for 15 s. The experiment was repeated thrice.
S. litura Hsp83 forward: AAACTGGCTGACTTGCTCCG
S. litura Hsp83 reverse: AACCACGCTTCTTGACTCGC
S. litura Krüppel homolog 1 forward: GGGACAACTGGTCATCCATC
S. litura Krüppel homolog 1 reverse: ACTGTGCAACGGTAACAACG
S. litura rp49 forward: AAGACCCGTCACATGCTACC
S. litura rp49 reverse: AGAATTGGTGACCCTGATGC
Results
Establishment of tobacco cutworm JH inhibitory activity analysis system and determining JHA specificity
Met and SRC sequences of tobacco cutworm were obtained from larvae through RNA-seq and de novo transcriptome assembly to construct a species-specific JHD analysis system for tobacco cutworm. The cDNA sequence encoding the full length of the ORF of Met was inserted into the yeast two-hybrid bait plasmid, and the cDNA sequence encoding the partial ORF was cloned into the yeast two-hybrid prey plasmid. The specificity of JH and JHA to the Met–SRC receptor complex of tobacco cutworm was confirmed using a tobacco cutworm analysis system. The Met–SRC complex was bound to JH, and the binding was JH- and JHA-dependent, except for methoprene and kinoprene, which acted as JHAs at 0.01 ppm concentration. The highest affinity was obtained with JH III (Fig. 1).
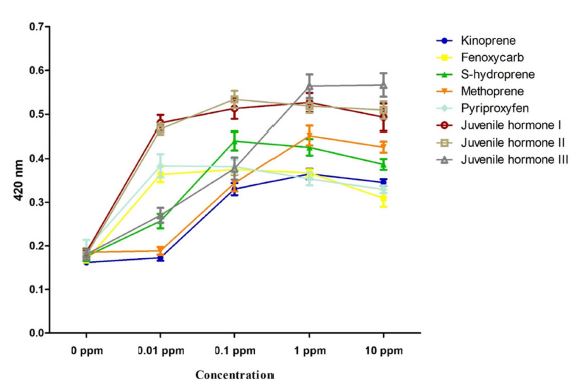
Microplate simulation of juvenile hormone III (JH III)- or juvenile hormone analog (JHA)-dependent methoprene-tolerant (Met)–steroid receptor coactivator (SRC) binding in Spodoptera litura. The JH/JHA-mediated binding of Met and SRC was measured as OD420 values after assaying β-galactosidase activity. Values and error bars indicate means ± SD (n = 3).
JHD activity of plant extracts
JHD activity tests were performed using the JHD assay system of tobacco cutworm with 1,628 plant extracts that were extracted with methanol. Based on the results, values ranging from 0.1 or less (without JHD activity) to 1.0 or higher (with high JHD activity) were identified. Based on this, average values of JHD activity of plant groups classified as angiosperms, gymnosperms, and spore-bearing plants were compared to check whether the JHD activity of plant extracts showed differentiation according to the taxa. Gymnosperms showed high average values. However, 20 plant extracts with the highest JHD activity generally belonged to angiosperms (Fig. 2A). In addition, upon comparing the average values by selecting families that included more than 10 plant species, plant families with high mean values of JHD activity were identified as Cupressaceae, Pinaceae, and Araliaceae (Fig. 2C). Among the 20 plant extracts with the highest JHD activity, gladiolus extract showed the highest JHD activity, and extracts from Camellia, Hosta longipes, and Lindera erythrocarpa exhibited high JHD activity by virtue of disrupting the binding of the Met–SRC complex in tobacco cutworm (Fig. 2B).
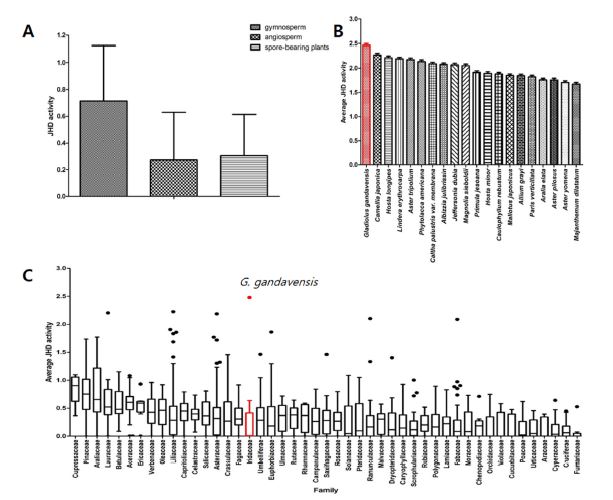
Distribution of juvenile hormone disruptor (JHD) activity among vascular plants. The JHD activities of 1,628 plant species were screened using Spodoptera litura Met–SRC binding assay systems. A: Mean JHD activities of three major plant groups. B: Mean JHD activities of 20 plant species that exhibited significantly high JHD activity in S. litura. C: Average JHD activity value for each plant family including more than 10 species. Values and error bars indicate means ± SEM; p < 0.0001 (t-test).
Changes in emergence rates and body weights of tobacco cutworm larvae according to feeding
To proceed with the bioassay of tobacco cutworm, we confirmed that the growth of larvae was inhibited when gladiolus extract, which showed the highest JHD activity in the JHD assay system, was mixed with artificial feed (Supplementary Figs. 1–3). Although there were differences in b ody w eig hts as t he l arvae g rew in t he c ontrol g roup (artificial feed) and negative control group (red pepper extract 10% + artificial feed), 77.7% and 83.3% of the larvae in each group grew normally, respectively. However, larvae fed artificial feed containing 10% gladiolus extract did not grow normally. Therefore, the larvae of each instar stage compared to the larvae in the control group showed a significant reduction in body weight increase (Fig. 3), and the larvae did not grow properly as pupae, resulting in a 0% rate of adult emergence (Table 1). To investigate the effect of gladiolus extract on the expression of JH-induced genes in tobacco cutworm larvae, qPCR was performed on the gene expression of Krüppel homolog 1 (Kr-h1), known as a JH subgene, and heat shock protein 83 (Hsp83), t he g ene encoding a protein bound to the JH response region in the presence of JH, in larvae fed artificial feed mixed with gladiolus extract. The expression levels of both genes were lower than that in the control group in the case of larvae fed the artificial feed mixed with gladiolus extract (Fig. 4).
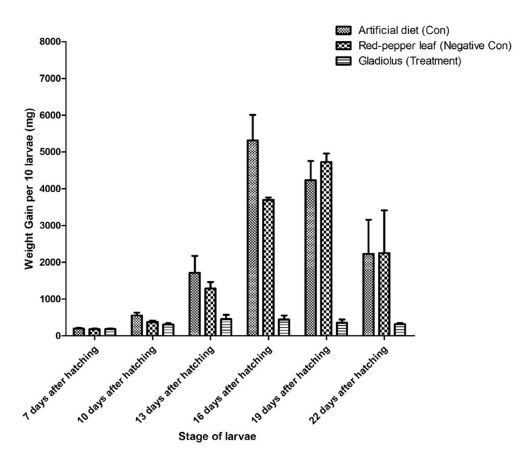
Effect of gladiolus extract on the feeding and growth of Spodoptera litura larvae. Effect of gladiolus on weight gain per 10 larvae during a 3-week feeding period. The artificial feed was supplemented with 10% (w/w) gladiolus extract, 10% (w/w) red pepper leaf extract, or ethanol. Weight gain was calculated as the difference between the weight of 10 larvae of each instar. Values and error bars indicate means ± SD (n = 3); p < 0.05 (t-test).

Average body weights and emergence rates of tobacco cutworm larvae by treatment group according to artificial feed containing gladiolus extract
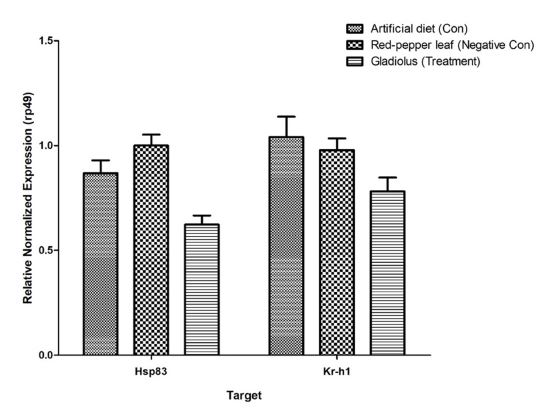
Validation of the juvenile hormone disruptor (JHD) activity gene from Spodoptera litura. JHD-dependent regulation of two genes: Krüppel homolog 1 (Kr-h1) and heat shock protein 83 (Hsp83). Transcript levels were measured by quantitative polymerase chain reaction (qPCR). Values and error bars indicate means ± SD (n = 3); p < 0.05 (t-test).
Discussion
Insects and plants have coexisted for 350 million years. Some are mutually beneficial, such as in pollination, but many insects have a predator–host relationship, whereby insect prey on plants and are adapted to withstand plant defense mechanisms (Gatehouse, 2002; Harborne, 1988). Plants have invested in the production of various organic compounds called secondary metabolites for defenses and other purposes. These secondary metabolites have undergone repeated evolutionary selection and are involved in attracting pollinators or conferring insecticidal or repellent properties (Wink, 2018). Among such secondary metabolites, plant-derived IGRs are considered an ideal defense tactic for plants because they act specifically on insects and are known to be non-toxic to plants themselves (War et al., 2020). In addition, such plant-derived substances interfere with the development of insect larvae by generating JHDs (Oh et al., 2017). Therefore, we established an in vitro screening system using yeast cells transformed with Met (the JH receptor of tobacco cutworm) and SRC to screen promising plant extracts.
Using the established screening system, 67 plant extracts with high activity were identified. The repellent and insecticidal activity of plant extracts of the Araliaceae, Pinaceae, and Cupressaceae families, which show high activity against mosquito larvae, were confirmed (Amer and Mehlhorn, 2006a, b, c; Barnard, 1999; Carroll et al., 2011; Giatropoulos et al., 2013; Prajapati et al., 2005; Sedaghat et al., 2011; Trongtokit et al., 2005; Vourlioti-Arapi et al., 2012). Gymnosperms, which showed high average activity for each taxon, might possess high JHD activity because defense measures that developed hundreds of millions of years ago against herbivorous insects continue to evolve (Shin et al., 2018b).
As in other insects, JH is essential for regulating the development, reproduction, and behavior throughout larval life of the tobacco cutworm (Jindra et al., 2015; Jindra et al., 2013; Tojo et al., 1985). However, it has been reported that JHA, which has a function similar to that of JH, blocks normal larval-to-pupal development and shortens the lifespan and spawning period for the imago (Xu et al., 2015). Drosophila melanogaster larvae fed with JHA develop into pupae but fail to break out of the pupae. Drosophila larvae that feed on JHD die during development, and thus, the growth of normal larvae is inhibited. (Shin et al., 2018a). Similarly, the larvae of tobacco cutworm fed artificial feed mixed with 10% gladiolus extract did not develop into pupae and remained as larvae, ultimately dying.
The early JH-inducible genes Kr-h1 and Hsp83 are activated by the JH/Met/SRC complex, which plays an important role in the inhibition of insect metamorphosis (Li et al., 2019; Shin et al., 2018a), and their expression level was significantly decreased in larvae fed artificial feed mixed with 10% gladiolus extract (Fig. 4). This indicates that gladiolus extract interferes with the development of normally functioning JH by interfering with JH-mediated gene regulation. Therefore, the gladiolus extract selected through this study can be developed into eco-friendly pesticides in the future.
Acknowledgments
This research was supported by the National Institute of Agricultural Sciences (PJ 0148452021) and the National Research Foundation of Korea Research Initiative Program (NRF-2019R1A2C1003571).
Conflict of Interest
The authors declare that there are no conflicts of interest
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
-
Ahn KS, Yoon C, Kim KH, Nam SY, Oh MG, et al., 2013. Evaluation of acute and residual toxicity of insecticides registered on strawberry against honeybee (Apis mellifera). Korean J. Pestic. Sci. 17(3):185-192.
[https://doi.org/10.7585/kjps.2013.17.3.185]

-
Amer A, Mehlhorn H, 2006a. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 99(4):466-472.
[https://doi.org/10.1007/s00436-006-0182-3]

-
Amer A, Mehlhorn H, 2006b. Persistency of larvicidal effects of plant oil extracts under different storage conditions. Parasitol. Res. 99(4):473-477.
[https://doi.org/10.1007/s00436-006-0183-2]

-
Amer A, Mehlhorn H, 2006c. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 99(4):478-490.
[https://doi.org/10.1007/s00436-006-0184-1]

-
Ashok M, Turner C, Wilson TG, 1998. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc. Natl. Acad. Sci. USA. 95(6):2761-2766.
[https://doi.org/10.1073/pnas.95.6.2761]

- Bae S, Park K, 1999. Effects of temperature and food source on pupal development, adult longevity and oviposition of the tobacco cutworm, Spodoptera litura Fabricius. Korean J. Appl. Entomol. 38(1):23-28.
-
Barnard DR, 1999. Repellency of essential oils to mosquitoes (Diptera: Culicidae). J. med. Entomol. 36(5):625-629.
[https://doi.org/10.1093/jmedent/36.5.625]

-
Carroll JF, Tabanca N, Kramer M, Elejalde NM, Wedge DE, et al., 2011. Essential oils of Cupressus funebris, Juniperus communis, and J. chinensis (Cupressaceae) as repellents against ticks (Acari: Ixodidae) and mosquitoes (Diptera: Culicidae) and as toxicants against mosquitoes. J. Vector Ecol. 36(2):258-268.
[https://doi.org/10.1111/j.1948-7134.2011.00166.x]

-
Gatehouse JA, 2002. Plant resistance towards insect herbivores: a dynamic interaction. New Phytol. 156(2):145-169.
[https://doi.org/10.1046/j.1469-8137.2002.00519.x]

-
Giatropoulos A, Pitarokili D, Papaioannou F, Papachristos DP, Koliopoulos G, et al., 2013. Essential oil composition, adult repellency and larvicidal activity of eight Cupressaceae species from Greece against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 112(3):1113-1123.
[https://doi.org/10.1007/s00436-012-3239-5]

- Goh H, Lee S, Lee B, Choi K, Kim J, 1990. Simple mass rearing of beet armyworm Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), on an artificial diet. Korean J. Appl. Entomol. 29(3):180-183.
-
Grabherr MG, Haas BJ, Yassour M, Levin JZ., Thompson DA., et al., 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 29(7):644-652.
[https://doi.org/10.1038/nbt.1883]

-
Han JH, Jeong HJ, Kim JY, Lee M, Kim D, et al., 2018. Selection and characterization of entomopathogenic fungus, Beauveria bassiana for the microbial control of Spodoptera litura. Korean J. Pestic. Sci. 22(4):269-275.
[https://doi.org/10.7585/kjps.2018.22.4.269]

-
Harborne JB, 1988. Flavonoids in the environment: structure-activity relationships. Prog Clin Biol Res. 280:17-27.
[https://doi.org/10.1007/978-1-4899-2913-6]

- Holloway J, 1989. The Moths of Borneo : family Noctuidae, trifine subfamilies : Noctuinae, Heliothinae, Hadeninae, Acronictinae, Amphipyrinae, Agaristinae. Malayan Nature Journal. 42:57-226.
-
Jindra M, Belles X, Shinoda T, 2015. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 11:39-46.
[https://doi.org/10.1016/j.cois.2015.08.004]

-
Jindra M, Palli SR, Riddiford LM, 2013. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58:181-204.
[https://doi.org/10.1146/annurev-ento-120811-153700]

- Jung SY, Seo MJ, Youn YN, Yu YM, 2010. Characteristics of endotoxin protein produced from Bacillus thuringiensis subsp. kurstaki KB099 isolate showing high bioactivity against Spodoptera litura. Korean J. Pestic. Sci. 14(4):446-455.
- Kahriman N, Yücel M, Yayli B, Arslan T, Alpay Karaoğlu, Ş, et al., 2012. Chemical composition and antimicrobial activity of the volatile of Gladiolus atroviolaceus Boiss. Asian Journal of Chemistry. 24:1461-1464.
-
Kim D-A, Kim J-S, Kil M-R, Paek SK., Choi SY, et al., 2008. Characterization of new Bacillus thuringiensis isolated with bioactivities to tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Korean J. Appl. Entomol. 47(1): 87-93.
[https://doi.org/10.5656/KSAE.2008.47.1.087]

-
Lee SH, Oh HW, Fang Y, An SB, Park DS, et al., 2015. Identification of plant compounds that disrupt the insect juvenile hormone receptor complex. Proc. Natl. Acad. Sci. USA. 112(6):1733-1738.
[https://doi.org/10.1073/pnas.1424386112]

-
Li K, Jia QQ, Li S, 2019. Juvenile hormone signaling–a mini review. Insect Sci. 26(4):600-606.
[https://doi.org/10.1111/1744-7917.12614]

- Malarvannan S, Murali P, S.P S, Vaiyapuri P, Nair S, 2010. Laboratory evaluation of the entomopathogenic fungi, Beauveria bassiana against the Tobacco caterpillar, Spodoptera litura Fabricius (Noctuidae: Lepidoptera). JBiopest.
-
Nauen R, 2007. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 63(7):628-633.
[https://doi.org/10.1002/ps.1406]

-
Ninkuu V, Zhang L, Yan J, Fu Z, Yang T, et al., 2021. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 22(11):5710.
[https://doi.org/10.3390/ijms22115710]

-
Oh HW, Yun CS, Jeon JH, Kim JA, Park DS, et al., 2017. Conifer diterpene resin scids disrupt juvenile hormone-mediated endocrine regulation in the Indian meal moth Plodia interpunctella. J Chem Ecol. 43(7):703-711.
[https://doi.org/10.1007/s10886-017-0861-9]

-
Palli S, Retnakaran A, 2001. Ecdysteroid and juvenile hormone receptors: properties and importance in developing novel insecticides. Biochemical sites of insecticide action and resistance: Springer. 107-132.
[https://doi.org/10.1007/978-3-642-59549-3_5]

-
Rivero A, Vezilier J, Weill M, Read AF, Gandon S, 2010. Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS pathogens. 6(8): e1001000.
[https://doi.org/10.1371/journal.ppat.1001000]

-
Prajapati V, Tripathi A, Aggarwal K, Khanuja S, 2005. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour Technol. 96(16):1749-1757.
[https://doi.org/10.1016/j.biortech.2005.01.007]

-
Sayyed AH, Ahmad M, Saleem MA, 2014. Cross-resistance and genetics of resistance to indoxacarb in Spodoptera litura (Lepidoptera: Noctuidae). J. Econ Entomol. 101(2):472-479.
[https://doi.org/10.1093/jee/101.2.472]

-
Sedaghat MM, Dehkordi AS, Khanavi M, Abai MR, Mohtarami F, et al., 2011. Chemical composition and larvicidal activity of essential oil of Cupressus arizonica EL Greene against malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Pharmacog Res. 3(2):135-139.
[https://doi.org/10.4103/0974-8490.81962]

-
Shin SW, Jeon JH, Jeong SA, Kim JA, Park DS, et al., 2018a. A plant diterpene counteracts juvenile hormone-mediated gene regulation during Drosophila melanogaster larval development. PLoS One. 13(7):e0200706.
[https://doi.org/10.1371/journal.pone.0200706]

-
Shin SW, Jeon JH, Yun CS, Jeong SA, Kim JA, et al., 2018b. Species-specific interactions between plant metabolites and insect juvenile hormone receptors. J Chem Ecol. 44(11): 1022-1029.
[https://doi.org/10.1007/s10886-018-1001-x]

- Smith CA, 1995. Searching for safe methods of flea control. J Am Vet Med Assoc. 206(8):1137-1143.
-
Tojo S, Morita M, Hiruma K, 1985. Effects of juvenile hormone on some phase characteristics in the common cutworm, Spodoptera litura. J. Insect Physiol. 31(3):243-249.
[https://doi.org/10.1016/0022-1910(85)90126-X]

-
Trongtokit Y, Rongsriyam Y, Komalamisra N, Apiwathnasorn C. 2005. Comparative repellency of 38 essential oils against mosquito bites. Phytother Res. 19(4):303-309.
[https://doi.org/10.1002/ptr.1637]

-
Vourlioti-Arapi F, Michaelakis A, Evergetis E, Koliopoulos G, Haroutounian S, 2012. Essential oils of indigenous in Greece six Juniperus taxa. Parasitol. Res. 110(5):1829-1839.
[https://doi.org/10.1007/s00436-011-2706-8]

-
War AR, Buhroo AA, Hussain B, Ahmad T, Nair RM et al., 2020. Plant defense and insect adaptation with reference to secondary metabolites. Co-Evolution of Secondary Metabolites: 795-822.
[https://doi.org/10.1007/978-3-319-96397-6_60]

-
Wilson TG, Ashok M, 1998. Insecticide resistance resulting from an absence of target-site gene product. Proc. Natl. Acad. Sci. USA. 95(24):14040-14044.
[https://doi.org/10.1073/pnas.95.24.14040]

-
Wink M, 2018. Plant secondary metabolites modulate insect behavior-Steps toward addiction. Front Physiol. 9:364.
[https://doi.org/10.3389/fphys.2018.00364]

-
Xu Q, Tang B, Zou Q, Zheng H, Liu X, et al., 2015. Effects of pyriproxyfen on female reproduction in the common cutworm, Spodoptera litura (F.)(Lepidoptera: Noctuidae). PLoS One. 10(10):e0138171.
[https://doi.org/10.1371/journal.pone.0138171]

Jun Hyoung Jeon: Biological Resource Center, Korea Research Institute of Bioscience and Biotechnology, Postdoctoral researcher, https://orcid.org/0000-0002-3784-6507
Seon-Ah Jeong: Biological Resource Center, Korea Research Institute of Bioscience and Biotechnology, Ph.D. student
1Ji-Ae Kim: Core Facility Management Center, Korea Research Institute of Bioscience and Biotechnology, Researcher
Doo-Sang Park: Biological Resource Center, Korea Research Institute of Bioscience and Biotechnology, Senior Researcher Ph,D
2Boyoon Seo: Crop Foundation Division, National Institute of Crop Science, RDA, Researcher Ph,D
1*Hyun-Woo Oh: Core Facility Management Center, Korea Research Institute of Bioscience and Biotechnology, Senior Researcher Ph,D https://orcid.org/0000-0003-3720-1896
Conceptualization: Jun Hyoung Jeon, Doo-Sang Park, Hyun-Woo Oh,Data curation: Jun Hyoung Jeon, Seon-Ah Jeong, Ji-Ae Kim, Boyoon Seo, Formal analysis: Jun Hyoung Jeon, Seon-Ah Jeong, Ji-Ae Kim, Doo-Sang Park, Boyoon Seo, Hyun-Woo Oh, Funding acquisition: Hyun-Woo Oh, Supervision: Hyun-Woo Oh, Doo-Sang Park, Writing – original draft: Jun Hyoung Jeon
