Development of a Residue Analysis Method for Metamifop in Paddy Water, Soil, and Rice with HPLC
Abstract
An analytical method for detecting metamifop residue in paddy water, soil, and rice with high performance liquid chromatography (HPLC) was developed. Water was extracted with ethyl acetate before analyzing by HPLC. Soil residues were extracted with acetone under acidic condition and after purifying with Extrelut® NT, and silica SPE, the residue was analyzed by HPLC. For residue analysis in rice, the procedure involved extraction with acetone, purification with Extrelut® NT, partitioning between acetonitrile/hexane, purification with silica SPE cartridge, and analysis by HPLC. The limit of detection (LOD) was 1.0 ng, limit of quantitation (LOQ) was 3.0 ng, and method limit of quantitation (MLOQ) were 0.001 mg/L for paddy water, 0.01 mg/kg for rice and soil, respectively. Standard calibration curve shows linearity from 0.05 mg/kg to 5.0 mg/kg (R2 = 0.9999). The recoveries in fortified paddy water were 91.3 ± 3.5% (0.01 mg/L level) and 93.2 ± 6.3% (0.05 mg/L level). The recoveries in fortified paddy soils were 92.5 ± 4.0% (0.1 mg/kg level) and 92.7 ± 4.0% (0.5 mg/kg level) in soil A, while, 102.3 ± 4.4% (0.1 mg/kg level) and 98.9 ± 7.9% (0.5 mg/kg level) in soil B, respectively. The recoveries in fortified rice were 93.0 ± 6.9% (0.1 mg/kg level) and 85.0 ± 3.5% (0.5 mg/kg level). This method was proved to be effective and can be used to determine the metamifop residue in paddy water, paddy soil, and rice.
초록
고성능 액체크로마토그래프(HPLC)를 이용한 농업용수, 논토양, 현미 중 잔류 metamifop을 분석하기 위한 최적화한 추출법과 정제법을 조합하여 분석법을 개발하였다. 농업용수 중 잔류량은 ethyl acetate로 분배 추출, 논토양 중 잔류량은 아세톤으로 추출하고, Extrelut® NT 흡착 정제 후 실리카 SPE로 정제하여 HPLC로 분석하였다. 현미 중 잔류량은 아세톤으로 추출하고, Extrelut® NT 흡착 정제과정과 acetonitrile/hexane 분배 후 실리카 SPE로 정제하여 HPLC로 분석하였다. 확립된 분석법의 검출 한계(limit of detection, LOD)는 1.0 ng이었고, 시료의 양을 고려한 방법정량한계(method limit of quantitation, MLOQ)는 농업용수, 논토양과 현미 각각 0.001 mg/L, 0.01 mg/kg이었다. 표준검량선은 0.05 mg/kg 농도에서 5.0 mg/kg 범위까지 직선성을 보였고 (R2= 0.9999), 시료 중 회수율은 0.01, 0.05 mg/L 수준으로 첨가된 농업용수에서 각각 91.3 ± 3.5, 93.2 ± 6.3%이었다. 0.1, 0.5 mg/kg 수준으로 첨가한 A토양시료 중 회수율은 각각 92.5 ± 4.0, 92.7 ± 4.0%이었고, B토양시료 중 회수율은 102.3 ± 4.4, 98.9 ± 7.9%로 확인되었다. 또한, 0.1, 0.5 mg/kg 수준으로 첨가한 현미시료 중 회수율은 각각 93.0 ± 6.9, 85.0 ± 3.5%로 확립된 분석법은 농업용수, 논토양, 그리고 현미중 잔류 metamifop의 분석에 적용할 수 있을 것이다.
Keywords:
analysis, herbicide, HPLC, metamifop, residue키워드:
분석, 제초제, 고성능액체 크로마토그래프, 메타미포프, 잔류Introduction
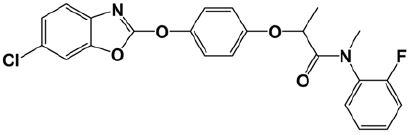
Metamifop ((R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy) phenoxy]-2’-fluoro-N-methyl propionanilide, CAS RN : 256412-89-2, Tomlin, 2009, Fig. 1) is a herbicide developed by Dongbu Hannong Chemical, Korea (Kim et al., 2003). It is highly selective between rice and barnyard grass, and used for post-emergence control of annual and perennial grass weeds in various crops, applying at 100-200 g/ha for rice (Chang et al., 2004). This chemical inhibits acetyl-CoA carboxylase (ACCase) like other AOPP (Aryloxyphenoxypropionicacid) series herbicides such as fenoxaprop, fenoxaprop- P, quizalofop and quizalofop-P (Tomlin, 2009).
Metamifop was registered in Korea to control annual weed Echinochloa utilis in paddy field. By combination with other herbicides metamifop is used to kill annual weeds such as Echinochloa utilis, Bidens tripartite L., monochoria vaginalis, Lindernia procumbens, Ludwigia prostrate, or perennial weeds such as Sagittaria trifolia, Eleocharis kuroguwai, Scirpus juncoides var. hotarui (Ohwi) Ohwi. (KCPA, 2016). This herbicide has been exported to China, Indonesia, Philippines, and Japan since 2010 (Choi et al., 2011).
In most cases these AOPPs herbicides effectively control weeds at low concentrations with low toxicity for nontarget species and have reduced persistence in the soil (Somers, 1996). The acute oral LD50 (rat) of metamifop is > 2,000 mg/kg and acute percutaneous LD50 (rat) is > 2,000 mg/kg, indicative of low mammalian toxicity (Tomlin, 2009).
Pesticides residues remaining on the food crop after spray or a contaminated food can result in harmful effects for the consumer and the environment. Therefore, quantification of the level of residue in crop, food and environmental samples is essential for consumer and environmental safety.
Moon et al. (2010) analyzed metabolite of metamifop from photochemical reactions and Park (2004) developed HPLC analysis conditions for metamifop and the metabolites from microsomal reactions. These methods adapted the direct extraction of the reaction mixture with ethyl acetate or dichloromethane without further purification with SPE or column chromatography. So these methods would not be suitable for residue analysis of agricultural or environmental samples containing many interfering substances. And enzyme linked immunosorbent assay (ELISA) of metamifop was studied with only buffer solution have not been adapted to real samples (Moon et al., 2007).
The purpose of this study is the establishment of metamifop residues analysis method from paddy water, paddy soil, and rice for routine residue analysis. Optimization of clean up, partition procedure, extraction, and method validation were carried out to develop precise and reliable analytical method.
Materials and Methods
Chemical and Reagents
Metamifop (purity 99.2%) was kindly donated by Dongbu Hannong Chemical. All solvents used for HPLC analysis were HPLC grade (Fisher, USA). SPE cartridge containing 1 g of silica or florisil was purchased from Alltech (Alltech BioKorea, Korea). Extrelut® NT resin was from Merck (Merck South Korea, Korea). All the other reagents and common chemicals were reagent grade or better.
Paddy water samples were collected from Seoul National University Farm (Suwon, Korea) and filtered through a 0.2 μm membrane filter. Paddy soils were collected from Seoul National University Farm (Soil A, Suwon, Korea) or Gimpo (Soil B, Gimpo, Gyunggi, Korea) and air dried, sieved with 10 mesh sieve. Physicochemical properties of used soil were shown in Table 1. Rice was grown in Seoul National University Farm and were hulled after harvest.
HPLC System
HPLC analysis was performed using an HP 1100 system (Hewlett-Packard, USA) with Luna C-18 column (4.6 × 250 mm, 5 μm, Phenomenex, USA). The mobile phase consisted of water and acetonitrile. A one step linear gradient [A: acetonitrile, B: water; 40% A, 60% B from 0 min to 3 min; 90% A, 10% B at 20 min; 90% A, 10% B at 30 min] was employed over 30 min with the flow rate of 1.0 mL/min. An aliquot of 20 μL was analyzed and UV detection was performed at 238 nm with diode array detector.
Standard Calibration Curve
Metamifop analytical standard (99.2%, 10.1 mg) was dissolved in 100 mL acetonitrile to make stock solution. From this stock solution, standard solutions of 5.0, 2.5, 1.0, 0.5, 0.2, 0.1, 0.05 μg/mL were prepared by serial dilution with acetonitrile. An aliquot of 20 μL was analyzed with HPLC and the standard calibration curve was prepared based on the peak area. The limit of detection (LOD) was calculated as 3 times the signal to noise ratio, and the limit of quantitation (LOQ) was calculated as 10 times the signal to noise ratio (Lee et al., 2013).
Establishment of Purification or Partition Method
Standard solution of metamifop in 5% ethyl acetate/hexane (10 mg/L, 1 mL) was loaded on to silica or florisil® SPE cartridges, that were previously conditioned with 5 mL hexane, and then the cartridge was eluted with 10 mL solution of 5%, 10%, 20%, 30%, 40%, and 50% ethyl acetate in hexane. After each eluate was evaporated with a gentle stream of nitrogen, the residue was dissolved in 2 mL of acetonitrile and analyzed with HPLC.
Standard solution of metamifop in acetonitrile (10 mg/L, 1 mL) was added to 20 mL of water and loaded to a column packed with 20 g of Extrelut® NT resin. After 30 min, the column was eluted with 150 mL of extraction solvent such as ethyl acetate, hexane, or dichloromethane. After solvent was evaporated under reduced pressure, the residue was dissolved in 2 mL acetonitrile and analyzed with HPLC.
Standard solution of metamifop in acetonitrile (10 mg/L, 1 mL) was added to 50 mL of acetonitrile saturated with hexane and extracted with hexane saturated with acetonitrile twice (50 mL + 50 mL). After each hexane and acetonitrile fraction was evaporated under reduced pressure, the residue was dissolved in 2 mL acetonitrile and analyzed with HPLC.
Standard solution of metamifop in acetonitrile (10 mg/L, 1 mL) was added to 100 mL water and extracted with a different solvent system (I, II, III and IV) twice (100 mL + 100 mL) (I; ethyl acetate, II; saturated sodium chloride (10 mL) solution + ethyl acetate, III; dichloromethane and IV; saturated sodium chloride (10 mL) solution + dichloromethane). After each organic solvent fraction was evaporated under reduced pressure, the residue was dissolved in 2 mL acetonitrile and analyzed with HPLC.
Recovery Tests of Metamifop from Samples
Paddy water (100 mL) fortified with metamifop at 0.01 and 0.05 mg/L levels were extracted with ethyl acetate twice (100 mL + 100 mL) and the organic layer was dried over anhydrous sodium sulfate. After solvent was evaporated, the resulting residue was dissolved in 2 mL acetonitrile and analyzed with HPLC.
HCl solution (0.1 N, 10 mL) was added to paddy soil (10 g) fortified with metamifop to reach 0.1 and 0.5 mg/kg, and extracted with acetone (100 mL + 50mL). After eliminating acetone with an evaporator under reduced pressure, the aqueous fraction was loaded on a column packed with 20 g of Extrelut® resin. After 30 min, the column was eluted with 150 mL ethyl acetate. After solvent was evaporated, the residue in 2 mL of 10% ethyl acetate in hexane was loaded on a silica SPE cartridge conditioned with 5 mL hexane. The elution fraction with 15% ethyl acetate in hexane (10 mL) was discarded and metamifop was eluted with 10 mL of 30% ethyl acetate in hexane. After solvent was evaporated with gentle stream of nitrogen, the residue dissolved in 2 mL acetonitrile, and analyzed with HPLC.
Water (10 mL) was added to rice (10 g) sample which was fortified with metamifop at the concentrations of 0.1 and 0.5 mg/kg and then the sample was extracted with acetone twice (100 mL + 50 mL) after 1 hr. After removing acetone under reduced pressure, the residual aqueous fraction was loaded on a column packed with 20 g of Extrelut® resin. After 30 min, the metamifop was eluted with 150 mL ethyl acetate. Solvent was evaporated under reduced pressure. The residue was dissolved in 50 mL acetonitrile which saturated with hexane and extracted twice with 50 mL hexane which saturated with acetonitrile. The acetonitrile layer was collected, evaporated, and dissolved in 2 mL of 10% ethyl acetate in hexane and loaded on a silica SPE cartridge conditioned with 5 mL of hexane. The elution fraction with 15% ethyl acetate in hexane (10 mL) was discarded and the metamifop was eluted with 10 mL of 30% ethyl acetate in hexane. After solvent was evaporated with gentle stream of nitrogen, the residue was redissolved in 2 mL acetonitrile and analyzed with HPLC.
Results and Discussions
Standard Calibration Curve
The retention time of metamifop on the HPLC operating under the conditions described in the methods section was 18.8 min, the limit of detection (LOD) was 1.0 ng, and the calibration curve showed good correlation (R2= 0.9999) linearity from 1.0 ng to 100 ng.
Purification with SPE Cartridge
When metamifop was purified through silica or Florisil® SPE cartridge, 10 mL of 30% ethyl acetate in hexane from the silica SPE gave best recovery (103.0%) while 10 mL of 20% ethyl acetate in hexane and 30% ethyl acetate in hexane from the Florisil® SPE gave 53.4% of recovery and 50.0% of recovery, respectively (Table 2). Therefore, a silica SPE and 10 mL of 30% ethyl acetate in hexane was selected for purification of metamifop from various sample matrices.
Extraction from Extrelut®
Extrelut® column was used for soil and rice in this study because it is designed as solid phase replacements for traditional liquid-liquid extractions with a separatory funnel. It contains specially processed wide-pore diatomaceous earth, has been used for determination of PAH metabolite levels in urine collected from workers exposed to PAH (Grimmer et al., 1993), Organophosphorous insecticide in vegetable oils (Muccio et al., 2005), methomyl (Ito et al., 1998), food preservatives (Leuenberger et al., 1979), morpholine in the human heart and kidney (Kudo et al., 2006), and terpenes from Ginkgo extract (Pietta et al., 1990).
To check the extraction efficiency of metamifop by Extrelut® NT resin, metamifop in water was loaded on the resin and eluted with 150 mL of various organic solvent. Ninety eight percent, 70.5%, and 88.7% of metamifop was eluted with ethyl acetate, hexane, and dichloromethane, respectively. Therefore, ethyl acetate was adequate solvent for extraction of metamifop by using Extrelut® (Table 3).
Extraction with Liquid-Liquid Partition (LLP)
Metamifop dissolved in water was extracted with various organic solvents. Metamifop partitioned into organic solvent well, and saturated sodium chloride solution didn’t improve extraction efficiency (Table 4). Ethyl acetate was the most suitable solvent for LLP of metamifop from water.
Partition with Acetonitrile and Hexane
Because fats in high lipid samples such as oil, beans, and cereals can affect the performance of chromatography, fats should be removed before analysis. When chemicals are labile in acidic or alkaline conditions, a nondestructive method is preferred. Various methods are used such as solvent partitioning, alkaline treatment (saponification), and oxidative dehydration with sulfuric acid (Ahmed, 2003; Garcia-Reyes, 2007) to reduce the fat content in samples. Among these methods, only solvent partitioning is a nondestructive method. Metamifop has an amide part that can be affected by acidic or basic conditions, so solvent partitioning was used to remove lipid.
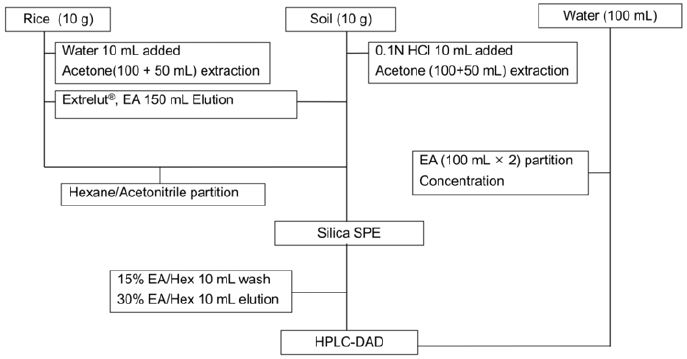
Therefore, partitioning with a mixture of acetonitrile and hexane was adopted to eliminate the lipid in rice samples in this study. Most of the metamifop (95.1%) remained in the actonitrile fraction (Table 4) after fats were removed by hexane. By combining those steps so far, the residue analysis scheme for metamifop in paddy water, paddy soil, and rice with HPLC was established (Fig. 2).
Recoveries of Metamifop from Various Sample Matrices
There were no interfering peaks in water extract (Fig. 3). The LOD was 1.0 ng, LOQ was 3.0 ng, MLOQ was 0.001 mg/L and recoveries were 91.3 ± 3.5% and 93.2 ± 6.3% at the fortified level of 0.01 mg/L and 0.05 mg/L, respectively. (Table 5)
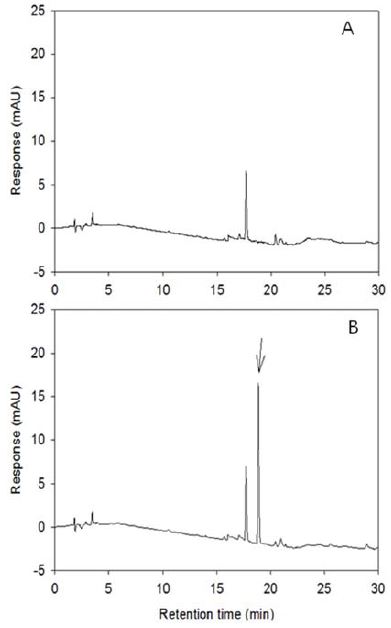
Typical chromatogram of metamifop residue analysis from paddy water. (A); untreated sample, (B); fortified at 0.5 mg/L.
Chromatogram of soil extract was quite clean (Fig. 4). The LOD was 1.0 ng, LOQ was 3.0 ng, MLOQ was 0.01 mg/kg and recoveries were 92.7 ± 4.3 and 92.5 ± 4.2% at the fortified level of 0.1 mg/kg and 0.5 mg/kg, respectively (Table 5). Celi et al. (1993) described a residue analysis method for the determination of fenoxaprop ethyl and fenoxaprop in soils using extraction with 0.1 M hydrochloric acid/methanol, partition with ethyl acetate, and alumina cartridge clean up. Recovery rates of metamifop from soil without hydrochloric acid were less than 70% (data not shown), but adding 0.1 N hydrochloric acid increased recovery rates to over 90%.
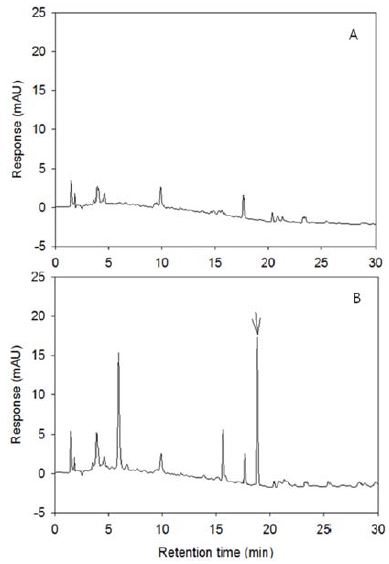
Typical chromatogram of metamifop residue analysis from paddy soil. (A); untreated soil A sample, (B); fortified at 0.5 mg/kg.
No interfering peaks were observed in rice extract (Fig. 5). The LOD was 1.0 ng, LOQ was 3.0 ng, MLOQ were 0.001 mg/L for paddy water and 0.01 mg/kg for soil and rice. Recoveries were 85.0 ± 4.1 and 93.0 ± 7.4% at the fortified level of 0.1 mg/kg and 0.5 mg/kg, respectively (Table 5).
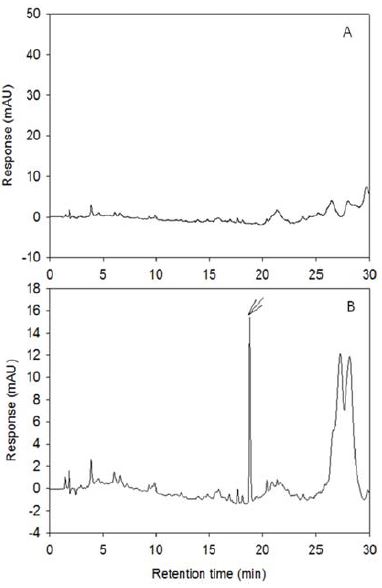
Typical chromatogram of metamifop residue analysis from rice. (A); untreated sample, (B); fortified at 0.5 mg/kg.
In conclusion, the sensitive and effective analytical method of metamifop residue in water, rice, and soil was developed. This method showed 1.0 ng of LOD, 3.0 ng of LOQ, 0.01 mg/kg of MLOQ, and 85.0 – 102.3% of recoveries from various samples, indicating the developed method can be used for routine residue analysis of metamifop.
References
-
Ahmed, F. E., (2003), Analysis of polychlorinated biphenyls in food products, Trends Anal. Chem, 22, p170-185.
[https://doi.org/10.1016/s0165-9936(03)00305-4]

-
Celi, L., M. Negre, and M. Gennari, (1993), HPLC determination of fenoxaprop and fenoxaprop-ethyl in different soils, Pest Manag. Sci, 38, p43-47.
[https://doi.org/10.1002/ps.2780380107]

- Chang, H.S., K. H. Chung, Y. K. Ko, J. W. Ryu, J. C. Woo, D. W. Koo, Y. H. Kang, T. J. Kim, J. S. Kim, B. J. Chung, O. Y. Kwon, and D. H. Kim, (2004), Synthesis and herbicidal activities of 2-[4-(6-chloro-2-benzoxazolyloxy)phenoxy]-propionamide derivatives, Korean J Pestic. Sci, 8, p145-149.
- Choi, J. S., C. M. Ryu, B.S. Han, D. H. Lee, and I. T. Hwang, (2011), Biochemical crop protecting agents for LOHAS, KIC News, 14, p29-40.
- Garcia-Reyes, J. F., C. Ferrer, M. J. Gomez-Ramos, A. Molina-Diaz, and A. R. Fernandez-Alba, (2007), Determination of pesticide residues in olive oil and olives, Trends Anal. Chem, 26, p239-251.
-
Grimmer, G., G. Dettbarn, and J. Jacob, (1993), Biomonitoring of polycyclic aromatic hydrocarbons in highly exposed coke plant workers by measurement of urinary phenanthrene and pyrene metabolites (phenols and dihydrodiols), Int. Arch. Occup. Environ. Health, 65, p189-199.
[https://doi.org/10.1007/bf00381155]

-
Ito, S., K. Kudo, T. Imamura, T. Suzuki, and N. Ikeda, (1998), Sensitive determination of methomyl in blood using gas chromatography-mass spectrometry as its oxime tertbutyldimethylsilyl derivative, J. Chromatogr. B, 713, p323-330.
[https://doi.org/10.1016/s0378-4347(98)00198-4]

- KCPA, (2016), Using guideline of crop protection agents, Korea Crop Protection Association, Samjung Inc, Seoul, Korea, p831.
- Kim, D. W., H. S. Chang, Y. K. Ko, J. W. Ryu, J. C. Woo, D. W. Koo, and J. S. Kim, (2003), Herbicidal phenoxypropionic acid N-alkyl-N-2-fluorophenyl amide compounds, US Patent 6600048.
-
Kudo, K., T. Ishida, N. Nishida, N. Yoshioka, H. Inoue, A. Tsuji, and N. Ikeda N, (2006), Simple and sensitive determination of free and total morphline in human liver and kidney using gas chromatography-mass spectrometry, J. Chromatogr. B, 830, p359-363.
[https://doi.org/10.1016/j.jchromb.2005.10.049]

- Lee, H., M. Riu, E. Kim, J. K. Moon, H. Choi, J. A. Do, J. H. Oh, K. S. Kwon, Y. D. Lee, and J. H. Kim, (2013), A single residue method for the determination of chlorpropham in representative crops using high performance liquid chromatography, J, Korean Soc. App. Biol. Chem, 56, p181-186.
-
Leuenberger, U., R. Gauch, and Baumgartner, (1979), Determination of food presevatives and saccharin by high-performance liquid chromatography, J. Chromatogr, 173, p343-348.
[https://doi.org/10.1016/s0021-9673(00)92302-1]

-
Moon, J. K., Y. S. Keum, E. C. Hwang, B. S. Park, H. R. Chang, Q. X. Li, and J. H. Kim, (2007), Hapten synthesis and antibody generation for a new herbicide, metamifop, J. Agric. Food Chem, 55, p5419-5422.
[https://doi.org/10.1021/jf070358f]

- Moon, J. K., J. H. Kim, and T. Shibamoto, (2010), Photodegradation pathway and mechanism of the herbicide metamifop in water/acetonitrile solution, J. Agric. Food Chem, 58, p12357-12365.
- Muccio, A. D., A. M. Cicero, A. Ausili, and S. D. Muccio, (2005), Determination of organophosphorus pesticide residues in vegetable oils by single-step multicartridge extraction and cleanup and by gas chromatography with flame photometric detector, Pesticide proctocols, Vol. 19, In Methods in biotechnology J. L. Martinez, and A. G. Frenich Eds, Humana Press Inc., Totowa, NJ, U.S.A, p263-271.
- Park, H. W., (2004), Metabolism of a new herbicide metamifop in rats, Thesis of mater degree, Seoul National University.
- Pietta, P. G., P. L. Mauri, and A. Rava, (1990), Analysis of terpens from Ginkgo Biloba L. extracts by reversed phase high-perormance liquid chromatography, Chromatographia, 29, p251-253.
- Somers, D. A., (1996), Aryloxyphenoxypropionate and cyclohexanedione resitant crops. In Herbicide resistant crops, S. O. Duke Eds, Lewis Publishers, Boca Raton, FL, U.S.A, p184.
- Tomlin, C. D. S., (2009), Metamifop, In A world compendium The Pesticide Manual 15th ED., C.D.S. Tomlin Eds, British Crop Protection Council, Alton, Hampshire, UK, p744.