Physicochemical and Biological Properties of Controlled-release Insecticide Formulation using Biodegradable Aliphatic Polyester Resin as Matrix
Abstract
This study was conducted to develop new formulations using biodegradable aliphatic polyester resin for the control of rose aphid, Macrosiphum ibarae, and cotton aphid, Aphis gossypii, under greenhouse condition. Imidacloprid [1-(6-chloro-3-pyridyl-methyl)-N-nitroimidazolidin-2-yldeneamine] was chosen as an active ingredient (a.i.) and two biodegradable aliphatic polyester resins were used as matrix. Two sheet formulations were formulated by heat-aided extrusion. Physicochemical properties and biological effect of the formulation on aphids were investigated. The incorporation rates of imidacloprid into the formulation were recorded 89.5 to 90.8%. Release rates of the imidacloprid from the formulations into water under static condition were remarkably affected by formulation type. The imidacloprid in the sheet formulation was rapidly released into water than that in the granular formulation. Degradation rates of biodegradable aliphatic polyester resin in the sheet formulations by soil-borne fungi during 45 days were 26.4 to 29.9%. But the resins were not degraded by bacteria as tested. Biological effects of the formulations on rose and cotton aphids were maintained over 90% even 180 days after application in the greenhouse. No phytotoxicity to the flowers was observed during the experimental period.
Keywords:
Aliphatic polyester resin, Aphid, Controlled release, Imidacloprid, Pesticide formulationIntroduction
The application of chemical pesticides is a general practice for the control of insects and diseases in agriculture. Among the pesticides, imidacloprid, [1-(6-chloro-3-pyridonylmethyl)-N-nitro-2-imidazolidinimine], is a systemic insecticide with novel modes of action (Kagabu et al., 1992; Moriya et al., 1992). Imidacloprid is a chloronicotinyl insecticide with properties that make it very attractive as a tool in integrated pest management. These properties include favorable environmental and toxicological characteristics, excellent systemic activity against a wide variety of insects on a wide range of crops, and a novel model of action that addresses the problem of pesticide resistance. It is an agonist of the nicotinergic acetylcholine receptor (Moffat, 1993; Mullins, 1993).
The Japanese Patent 08092006 (Showa Denko KK et al., 1996) discloses a biodegradable resin molded product containing a biologically active substance. The resin is produced by adding a biologically active agent such as a herbicide, an antifungal agent, an insecticide or a repellent to a biodegradable resin. The biologically active resin is preferably an aliphatic polyester resin produced by synthesizing a polyester prepolymer having relatively high molecular weight from a glycol and an aliphatic dibasic acid as main components and subsequently converting the prepolymer into a high polymer with a coupling agent. The US patent 03850862 (Union Carbide Corp., 1972) discloses a blend comprising biodegradable thermoplastic dialkanoyl polymers which are prepared by esterification technique using predetermined amounts of an aliphatic diol and an alkanedioic acid. The aliphatic glycol contains desirably from 2 to 8 carbon atoms and the alkanedioic acid contains desirably from 2 to 12 carbon atoms. Additional ingredients which can be included in the blend are plant nutrients, fertilizer, insecticides, pesticides, herbicides, and the like.
Usually, large amounts of pesticides are applied several times to get the desired pesticide effects such as prevention of breeding and extermination of the major pests in the nurseries. But the use of excessive amounts of pesticides causes problems such as hazards on other plants, pesticide users and health of crop consumers. It also causes serious environmental pollution. Distribution of pesticides in the market is also complicated and labor intensive. Conventional efforts have been done to solve these problems.
Controlled-release formulations have several advantages over conventional pesticide formulations, including lower application rates, ease and safety in handling, and reduced leaching potentials (Lewis and Cowsar, 1977; Bahadir and Pfister, 1990). Controlled-release formulations can ameliorate pesticide losses including those by leaching, evaporation, and degradation (Wilkins, 1990). Imidacloprid was incorporated into alginate granules by using calcium chloride as gallant to prepare a formulation to be used for controlled release (Gonzalez-Pradas et al., 1999).
This paper describes the development of a controlled-release pesticide formulation to extend efficacy against aphids using biodegradable polyester resin.
Materials and Methods
Instrumentation and reagent
Chromatographic determinations were performed on a HP-1100 Series equipped with UV absorbance detector (Agilent, California, USA). A stainless steel, 250 × 4.6 mm, 5 μm, 120Å C18 column (Merck, Darmstadt, Germany) was used at room temperature. HPLC conditions are as follows. Injection volume 10 μl, flow rate 1.0 ml/min, mobile phase water/acetonitrile (25/75, v/v), wavelength 275 nm.
Reagent
Water, acetonitrile, and dimethyl phthalate were purchased from Merck (Darmstadt, Germany).
Preparation of the Sheet formulation
Two sheet type formulations, ISF-I and II, were prepared. 98.6 g of aliphatic polyester resin and 1.4 g of imidacloprid was mixed using tumbler mixer. The mixture was passed through the twin screwed-extruder (Plasti-corder, Brabender, Germany) with cylinder temperatures ranging from 120 to 150oC. The formulation was passed through the cooling system. These were the form of stripe, 0.3 mm thick and 1.5 cm wide. The formulation recipes were shown in Table 1.
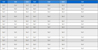
Quantities of active ingredient and inert materials used in the sheet formulations of imidacloprid for controlled release
Meanwhile, aliphatic polyester resins used were SG 2109 and SG 1111(SK Chemicals, Korea). The characteristics of SG 2109 and SG 1111 had melting points of 91oC and 113oC, intrinsic viscosities of 1.5 and 1.2, and average molecular weights of 31,000 and 25,000, respectively.
Availability of imidacloprid
About 5 g of sample was crushed using a freeze-crusher. 1.5 g of the crushed sample was placed into a 125 ml cap-stoppered Erlenmeyer flask and 100 ml of acetonitrile: distilled water (1 : 1, v/v) including 1000 ppm dimethyl phthalate as internal standard was added to the flask. The sample was extracted using ultrasonicator (Branson, USA) for one minute. The extract obtained was analyzed by High Performance Liquid Chromatograph (HPLC) and availability of imidacloprid was then determined on the basis of added value of imidacloprid at the stage of formulation.
Stability of imidacloprid in the formulations
The formulations were kept in an aluminum package that was sealed using the sealing machine. The package was stored at 50oC until a given number of days.
Dissipation rate of imidacloprid in the formulations were analyzed following the same procedure used in determining the availability imidacloprid with in a given number of days.
Biodegradation of the formulation in soil
A fixed amount of field soil was placed in a plastic container, 3 cm in diameter. A slice of the sheet formulations are treated in the container. The formulation was covered with small amount of soil
Soil moisture in the plastic container was adjusted to 10 and 60% of maximum water-holding capacity and the plastic container was kept at 50oC for 25 days. After 25 days, the sample was unpacked and carefully washed. Biodegradation rates were measured by weight loss of the formulations.
Release rate of imidacloprid from the formulation into water
The 125 ml cap-stoppered test tube was filled to 100 ml of distilled water. The formulations were then immersed in the water. Released amounts of imidacloprid from the formulation into water were analyzed after selected intervals using HPLC.
Biological effect against aphids and phytotoxicity of the formulation
The formulations were tested by burying them near the root zones of the rose and chrysanthemum plants. Target insect pests were rose aphids, Machrosiphum ibarae, on rose, Rosa hybrida and cotton aphids, Myzus persicae, on chrysanthemum, Dendranthema grandiflorum, under the greenhouse conditions.
Acephate WP was used as reference formulation and applied ten times during experiment. The number of control plot designates total counts of aphids in ten leaves. After treatment, the control effect and phytotoxicity of the formulations on target insect pests and rose plants were respectively investigated with lapse of time.
Results and Discussions
Availability of imidacloprid
As shown in Table 2, the availability of imidacloprid was more than 97%, which suggested thermolysis was negligible. The results suggested that the imidacloprid of the formulations was uniformly dispersed/incorporated on/in the sheet formulations.
Stability of imidacloprid in the formulations
Active ingredients of pesticides must be chemically stable during storage although there is no physical transformation of the product. It is annually a basic principle to analyze stability of a.i. in the pesticide formulations by storing the formulations for one year under natural room condition. This is to establish validity of the product (Tanaka, 1981). Generally, the results are analyzed through analogical interpretation using tests on the high temperature condition, because it is difficult to conduct tests over long period. Currently the recommended test durations are two, four, and six weeks in 54 ± 2oC by Manual on development and use of FAO and WHO specifications for pesticides (2010).
This experiment was conducted to determine the expiry date of the formulations. Samples were kept in a small ampoule with lid immediately sealed. The samples were stored at 50 ± 2oC until given period of time. The degradation rate of imidacloprid in the formulations recorded less than 1.0% even at 90 days after treatment (Table 3). The formulations were very stable during the experimental periods. Thus, these formulations do not need addition of special stabilizer to stabilize chemically, to extend their expiry dates. The forecasted expiry date of the formulations was more than three years.
Biodegradation of aliphatic polyester resin in soil
This study was conducted to know the pattern of degradation for the formulations applied on soil surface and in soil. The soil moisture was determined during the dry (usually from April to June and October to November) and rainy seasons (from July to September) in Korea.
A slice of the sheet formulations was treated on soil surface and in soil. The degradation was determined after 25 days. The formulation degradation was negligible in 10% water content regardless of site of application.
When the formulations were treated in 60% of water content, the degradation rate was more than 10%. The ISF-II formulation was degraded more 40% when treated in soil. ISF-II was degraded to more about 15% than ISF-I (Table 4). The difference of degradation between the two formulations was possibly caused by the different properties of polyester used. The biodegradable polyester resins are believed to be degraded by hydrolysis and soil-borne microorganisms.
Release rate of imidacloprid from the formulation into water
Elution ability of imidacloprid from the formulation has to gush out the pesticide incorporated with the resin by water. Through this, the formulation accomplishes its pesticidal actions. Pesticides that are not gushed out of the product lose their value as pesticides. Therefore, we can say that the elution ability of pesticides is very important.
The amounts of imidacloprid eluted into water were almost 100% at 25 days after treatment for both ISF-I and ISF-II formulations at 20 ± 1oC (Fig. 1).

Release profile of the insecticide imidacloprid from the sheet formulations into water under static condition.
In case of dispersing controlled release formulations mixed with polymer matrix, release rate of a.i. from formulation is up to concentration of a.i., dispensability, dissolution, and surface area of formulation in polymer matrix of formulation.
It is believed that release rate of a.i. from formulations used in this experiment were not effective due to several factors because the polyester resins used have different properties.
Biological effect of the formulation on aphids
As shown in Table 5 and 6, the control effects of the formulations tested against rose aphid, Machrosiphum ibarae and cotton aphids, Aphis gossypii, were more than 90% even at 173 to 200 days after treatment. Therefore, these results revealed equivalent or superior formulation compared with those applied with Acephate WP 10 times. This is an evidence of a controlled release formulation.

Biological effect of the sheet formulations to Aphis gossypii on Dendranthema grandiflorum in the greenhouse
No phytotoxicity to the plants was observed in using the recommended rate or doubled dosage of the formulation during the experimental period.
Acknowledgments
This study was financially supported by “Research Program for Agricultural Science & Technology Development (Project No. PJ010161)” National Institute of Agricultural Sciences, Rural Development Administration, Korea.
References
- Bahadir, M., and G. Pfister, (1990), Controlled release formulations of pesticides, In Haug G. Hoffmann H. (eds), Controlled Release, Biochemical Effects of Pesticides, Inhibition of Plant Pathogenic Fungi, Chemistry of Plant Protection, vol 6 Springer, Berlin, Heidelberg, p1-64.
- FAO/WHO Joint Meeting on Pesticide Specifications (JMPS), (2010), Manual on development and use of FAO and WHO specifications for pesticides, World Health Organization and Food and Agriculture Organization of The United Nations, p135.
- Kagabu, S., K. Moriya, K. Shibuya, Y. Hattori, S. Tsuboi, and K. Shiokawa, (1992), 1-(6-Halon-icotinyl)-2-nitromethyleneimidazolidines as Potential New Insecticides, Biosci. Biotechnol. Biochem, 56(2), p362-363.
- Lewis, D. H., and D. R. Cowsar, (1977), Principles of controlled release pesticides, In: Controlled Release Pesticides, H. B. Scher Eds, American Chemical Society, Washington DC, p1-16.
- Moffat, A. S., (1993), New Chemical Seek to Outwit Insect Pests, Science, 261(5121), p550-551.
- Moriya, K., K. Shibuya, Y. Hattori, S. Tsuboi, K. Shiokawa, and S. Kagabu, (1992), 1-(6-Chloro-nicotinyl)-2-nitromethyleneimidazolidines and Related Compounds as Potential New Insecticides, Biosci Biotechol Biochem, 56, p364-365.
- Mullins, J. W., (1993), Imidacloprid-A New Nitroguanidine Insecticide, In: Pest Control with Enhanced Environmental Safety, Duke, S. O., Menn, J. J., Plimmer, J. R. Eds, ACS Symposium Series 524, Americal Chemical Society, Washington DC, p183-198.
- Showa Denko KK and Showa Highpolymer Co. Ltd, (1996), Biodegradable resin molded product containing biologically active substance, Japanese Patent 08092006.
- Tanaka, F. S., R. G. Wein, and E. R. Mansager, (1981), Survey for surfactant effects on the photodegradation of herbicides in aqueous media, J. Agri. Food Chem., 29(2), p227-230.
- Union Carbide Corp., (1972), Blends of a biodegradable thermoplastic dialkanoyl polymer and naturally occurring biodegradable product, US patent 03850862.
- Wilkins, R. M., (1990), Controlled Delivery of Crop Protection Agents, Taylor and Francis, London, p149-165.