Characterizing the efficacy of potential insecticides to white-backed planthopper, Sogatella furcifera collected in the Republic of Korea (2020-2021)
Abstract
This study characterized the efficacy of seven insecticides against Sogatella furcifera Horváth (white-backed plant hoppers, WBPHs). WBPHs are known to frequently migrate from China or adjacent East Asian countries to the Korean Peninsula during spring and summer, causing destructive damage to rice crops by sucking cell sap from their leaves while rapidly increasing in population. Adult WBPH were sampled from the Republic of Korea’s western (Wanju and Seocheon) and southern (Namhae) regions in 2020 and 2021. Collected samples were cultured for two or three generations within an insect breeding room. Of the tested insecticides, etofenprox EC , fenobucarb EC, carbosulfan SC, dinotefuran WP, and imidacloprid SC all exhibited high to moderate mortality (75-93%) of the pests at 96 hrs post insecticide treatment, while pymetrozine WP and buprofezin WP showed low mortalities (< 45%) across all sampling locations. WBPH mortality rates did not differ significantly (p>0.05) between the western) and southern regions of South Korea. The findings of this study may help manage WBPHs more effectively in areas where they pose a significant challenge.
Keywords:
insecticidal efficacy, immigrant pest, monitoring, white-backed plant hopperIntroduction
Sogatella furcifera Horváth, white-backed planthopper (WBPH), is one of the most economically devastating insect species, along with brown plant hopper (BPH), Nilaparvata lugens because they damage rice paddies through direct sucking, oviposition, and virus disease transmission such as the southern rice black-streaked dwarf virus (SRBSDV) (Li et al., 2023). In contrast to BPH, the outbreak of WBPHs in the Republic of Korea (South Korea), is not as serious as in other neighboring countries such as China and Japan (Yin et al., 2017).
Few studies on insecticide resistance or monitoring of these two planthoppers have been conducted. Two species are unable to overwinter in South Korea, so they migrate from overwintering regions (Thailand, Vietnam, Laos, and China) each year, causing damage to rice production and endangering future food security. Since there are fewer than five insecticides registered for WBPHs and more than 100 registered for BPH, climate change may cause sudden outbreaks of WBPH in pest-friendly environments, as well as possible overwintering in greenhouse conditions.
In contrast of the frequent outbreaks of WBPHs in neighboring countries such as China or Japan (Liu et al., 2023; Matsumura, 2001; Matsumura et al., 2018), WBPH pest damage has not been considered as severe in South Korea when compared to BPH and small brown plant hopper (SBPH). This could be attributed to WBPH’s inability to overwinter in South Korea or other unidentified reasons. In the case of the sudden mass development of WBPHs, it may be challenging to take a preventive action against them due to the lack of registered pesticides. Due to the scarcity of registered pesticides, it may be difficult to take preventive measures against WBPHs if they suddenly occur.
China and Japan have conducted nationwide insecticide resistance monitoring of WBPHs (Huang et al., 2022; Matsumura et al., 2018). Since the 1980s , the size of WBPH population in China has increased annually, WBPH outbreaks have become more frequent, and WBPHs damage to rice plants has become a major challenge (Zhang et al., 2016). Since 2009, SRBSDV, a member of the Fijivirus genus that is transmitted via WBPHs (Zhou et al., 2008), has become prevalent in Chin. Chemical pesticides have become a major pest control method in China due to WBPH’s rapid overlapping generations within the crop life cycle, complicated migration sources, fast growing rate, spreading capability, and frequency of occurrence. WBPHs have been reported to be resistant to a wide range of conventional insecticides in China, including buprofezin, dinotefuran, fipronil, imidacloprid, and carbamates (Jianya et al., 2013; Li et al., 2021; Liu et al., 2023; Mu et al., 2016). A recent study monitored the resistance levels of WBPH field populations (2011 to 2021) collected in Central China (Anhui , Hubei, Hunan, and Henan Province) from 2011 to 2021, and found moderate levels of resistance to dinotefuran and etofenprox (Huang et al., 2022).
A previous study of insecticide susceptibilities in BPHs and WBPHs populations in Japan found that two rice planthoppers (RPHs) outbreaks fluctuated year after year in 1970’s and 1980’s (Matsumura, 2014; Otuka et al., 2009). When rice-seedlings boxes were treated with neonicotinoid and phenylpyrazole insecticides (e.i., imidacloprid and fipronil), the two species’ abundance decreased (Matsumura, 2014). However, simultaneous outbreaks of BPHs in China, Japan in 2005 and 2006, and Vietnam in 2006 and 2007 were mainly caused by the development of insecticide resistance to imidacloprid (Matsumura et al., 2008). These studies indicated that excessive application of a single pesticide can lead to more serious issues, such as pest population growth. Therefore, more judicious and rational insecticides application in the rice fields is recommended. Monitoring insecticide resistance is also necessary for effective pest management and the exact causes of a control failure, such as development of insecticide resistance, improper application, and unexpected mass outbreak, must always be investigated.
This study was aimed at monitoring the response of the WBPHs collected from southern and western parts of South Korea in the years of 2020 and 2021 (Namhae) to seven selected insecticides.
MATERIALS AND METHODS
Collection site and its rearing of WBPHs
WBPHs were manually collected from rice fields in the southern and western South Korea in July 2020 and 2021. The sampling sites in 2020 were Wanju, Jeollabuk-do (site 1), Seocheon, Chungcheongnam-do (site 2), Jangheung, Jollanam-do (site 3), Shinan, Jeollanam-do (site 4), Yeosu, Jeollanam-do (site 5), Sacheon, Gyeoungsangnam-do (site 6), and in 2021, Namhae, Gyeoungsangnam-do (site 7). WBPHs were mainly collected around the planthoppers observational plots installed by the Agricultural Technology Center located in each sampling site (Table 1). The insects were cultured for two or three generation (based on the time of the insect life cycle) at the insect breeding room of the Division of Crop Protection, National Institute of Agriculture and Science (NAS) in Wanju, before being used for insecticide treatments. Insecticide-susceptible WBPHs strains raised without any insecticide exposure for more than 10 years at NAS’ insectarium was used as control.
Insecticides
With BPHs or SBPHs, the outbreak of WBPHs has caused significant problems in several rice growing countries including India, China, Japan, Thailand, South Pacific Islands, and northern Australia. The insect outbreak occurs on a regular basis, resulting in ‘hopper burn’ in severely infested paddy fields of susceptible varieties. Asian Food and Agriculture Cooperation Initiative (AFACI) countries selected seven insecticides (Table 2) from insecticides lists on the markets in East Asian countries (Thailand, Vietnam, Myanmar, Cambodian, and Laos) to establish standard systems with the Rural Department Administration (RDA, 2012). In South Korea, 24 insecticides are commercially registered for WBPHs, but actual insecticides are clothianidin, fenobucarb, chlorantraniliprole, thiacloprid, acephate, etofenprox, tetraniliprole, phenthoate, and flubendiamide. Some commercial insecticides (Table 5) were selected and tested on the WBPHs collected in 2021.
Bioassay
The rice seedling dipping method, slightly modified by Jeong et al. 2017, was used to compare both years in this study. The Sindongjin rice seedlings used in the experiments were grown in a pot at 25 ± 2°C, 50-60% humidity, and a photoperiod of 16:8 (L:D) at NAS. The seedlings from the pot were collected and washed. After drying in the shade at room temperature, the seedling stems (7 cm) were cut without causing any damage to the shoot roots. The volume of each test insecticide concentration was large enough to allow the entire plants (over 4 week-seedlings) to be dipped, so 300-400 ml of each insecticide solution was prepared by diluting each insecticide product to the recommended concentration (the Korean market products). The root parts of five stems grouped were soaked for 30 s in each diluted insecticide before being air dried in a shade. To prevent moisture loss during the experiment, each pack of treated seedling was wrapped in sanitary cotton before being placed in glass tube (Ø 27 × h 200 mm). WBPHs (3 to 4 instars) were collected from the holding cage using a suction device and thirty WBPHs (n=3) were placed in each tube containing seedlings, with the cap loosely covered with wet sanitary cotton. These tubes were placed in the incubator under the same conditions as in which rice seedlings were raised. During the experiment time, water was sprayed onto the cotton every day. To assess mortalities, the number of dead and live insects was counted at 24, 48, 72, and 96 hrs after application of each insecticide. The bioassay experiment results were analyzed, and the analysis of variance (ANOVA) was conducted using SPSS 26 (IBM SPSS Statistics).
The insecticidal effect on WBPHs was calculated based on the formula ABBOTT (1925),
Ca is the amount of survival WBPH in no treatment (control) and Ta is the amount of survival WBPH in treated dish.
Results and Discussion
Responses of WBPHs (2020 and 2021) in South Korea to seven insecticides
Susceptibility of WBPHs to seven insecticides (etofenprox EC, fenobucarb EC, carbosulfan SC, dinotefuran WP, imidacloprid SC, pymetrozine WP, and buprofezin WP) are shown in Fig. 1. At 96 hours after insecticide treatment, mortality rates were as follows: fenobucarb (95.2 ± 4.7%), dinotefuran (92.7 ± 4.6%), carbosulfan (90.7 ± 6.9%), imidacloprid (79.7 ± 6.2%), etofenprox (78.6 ± 9.3%), pymetrozine (41.3 ± 13.2%), and buprofezin (36.0 ± 16.6%) (Fig. 1).
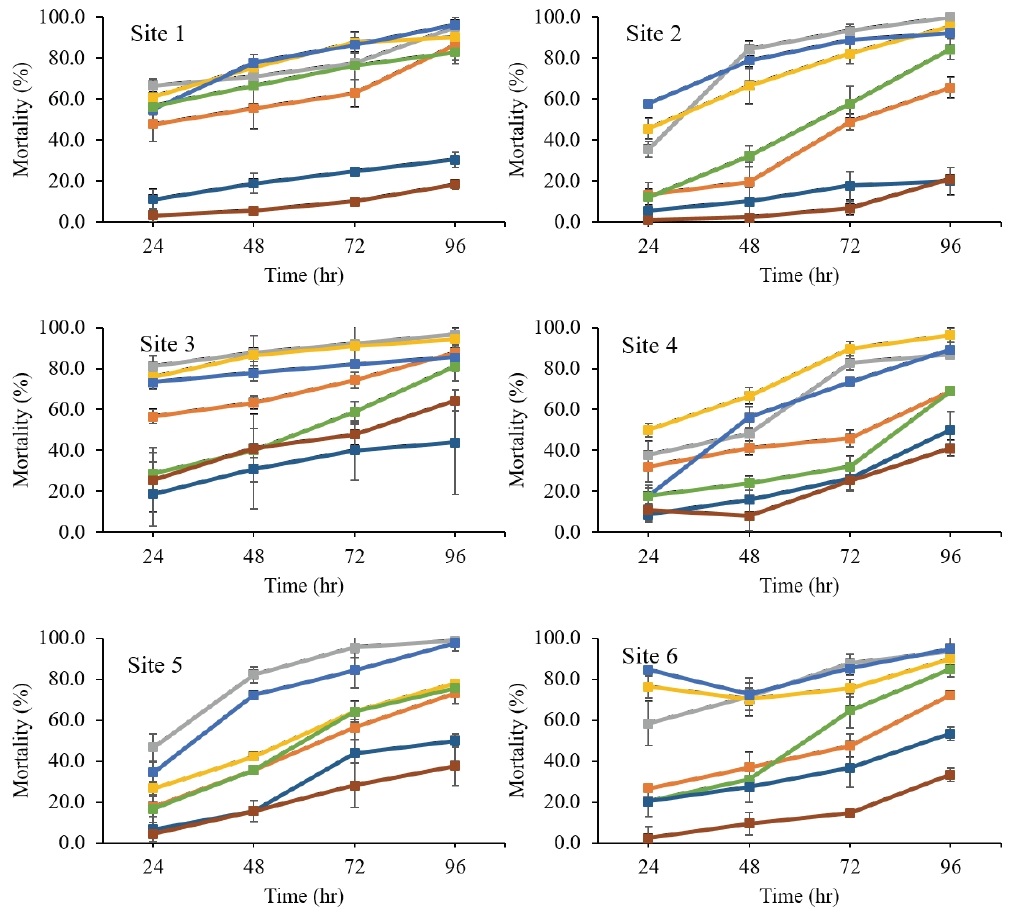
Susceptibility of white–backed plant hoppers (WBPHs) to 7 pesticides including etofenprox EC (■), fenobucarb EC (■), carbosulfan SC (■), dinotefuran WP (■), imidacloprid SC (■), pymetrozine WP (■), and buprofezin WP (■). The WBPHs were collected in 2020. Wanju, Jeollabuk-do (site 1), Seocheon, Chungcheongnam-do (site 2), Jangheung, Jeollanam-do (site 3), Shinan, Jeollanam-do (site 4), Yeosu, Jeollanam-do (site 5), and Sacheon, Gyeoungsangnam-do (site 6).
The results from Namhae (Fig. 2) show that fenobucarb (92.6%) is followed by dinotefuran (91.4%), carbosulfan (91.4%), imidacloprid (82.7%), etofenprox (79.0%), pymetrozine (32.1%), and buprofezin (29.6%). Sites 1 (Wanju) and 2 (Seocheon) are close together on South Korea's western coast (middle). Sites 3 (Jangheung), 4 (Shinan), 5 (Yeosu), and 6 (Sacheon) are all close to the southern seaside (Fig. 1). WBPH mortality rates did not differ significantly (p>0.05) between the western (Wanju and Seocheon) and southern regions of South Korea. Moreover, there is no significant difference in mortality rates between the southern sites (p>0.05).
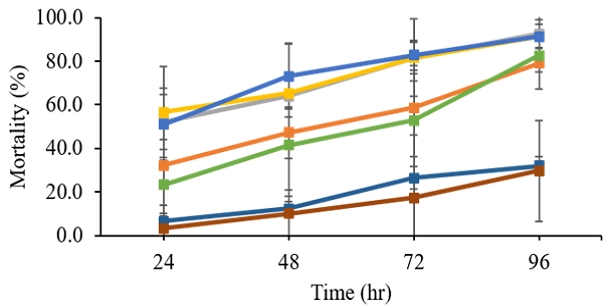
Susceptibility of white–backed plant hoppers (WBPHs) from Namhae, Gyeoungsangnam-do in 2021 to 7 pesticides including etofenprox EC (■), fenobucarb EC (■), carbosulfan SC (■), dinotefuran WP (■), imidacloprid SC (■), pymetrozine WP (■), and buprofezin WP (■).
Carbosulfan, dinotefuran, etofenprox, imidacloprid, and pymetrozine have not been officially registered for WBPHs in South Korea. Buprofezin is not registered for all the rice plant hoppers in South Korea, but pumetrozine and buprofezin are available to all in Cambodia, Thailand, and Vietnam (Park and Cho, 2020). Low average mortalities occurred on buprofezin WP (8.0% at 24 hrs, 13.7% at 48 hrs, 22.1 % at 72 hrs, and 36.0% at 96 hrs) and pymetrozine WP (11.9% at 24 hrs, 19.8% at 48 hrs, 31.6 % at 72 hrs, and 41.3% at 96 hrs) to WBPHs. Pymetrozine (pyridine azomethine compound) (Maienfisch, 2019) is a neuroactive (Ausborn et al., 2005) insecticide that disrupts the feeding behaviour of plant sucking insects and planthoppers, causing the pest to starve to death. Pymetrozine's action mode may be linked to the serotonin signalling pathway (Kaufmann et al., 2004). Buprofezin is an insect growth regulator (IGR) (De Cock and Degheele 1998). Pymetrozine and buprofezin have been shown to have slow-acting insecticidal effects. In this experiment, both insecticides did not show high mortality (less than 60%) of WBPHs 6 days after treatment, which is consistent with a study on insecticide responses of WBPHs collected in Shinan in 2020 in South Korea (Kang et al., 2022) and previous studies in China (Li et al., 2021). The previous study (Kang et al., 2022) demonstrated that pymetrozine WG (65.5%) resulted in low mortality. Based on these findings and several reports of buprofezin and pymetrozine resistance of WBPHs from China, the use of these two pesticides is not recommended on the spot. In addition, considering that WBPHs invades in South Korea mainly through the air current from southern China (Gao et al., 2020) and China from Myanmar, Laos, and Vietnam (Yin et al., 2017), we cannot rule out the possibility of two insecticide-resistance WBPHs being found in South Korea. According to Gao et al., 2020, it can be difficult to pinpoint the origin of BPHs and WBPHs that spread and mix together due to the air current movement.
Buprofezin replaced imidacloprid as the primary control insecticide for BPHs in China in the 2005s due to resistance (Wang et al., 2008), and it has been extensively used in the field since then. Previous study suggested that the use of buprofezin for WBPHs control in China was limited, and alternative insecticides with different modes of action were alternated with it to avoid further resistance development (Su et al., 2013). WBPHs resistance to buprofezin increased constantly from 2011 to 2013 (Zhang et al., 2014). Due to the severe resistance problem, the Chinese Ministry of Agriculture suspended its use for rice planthoppers control (RPHs) since 2014 (Wu et al., 2018). Despite the limited usage, field monitoring data from recent years showed insecticide resistance in the WBPH (Huang et al., 2022; Jin et al., 2017). One of the monitoring studies (Li et al., 2021) on the resistance level of WBPHs collected in Chinese rice paddy fields from 2019 to 2020, demonstrated that WBPHs were moderately to high resistance to buprofezin. Another study showed that about half of the field populations remained high resistance to buprofezin (Liu et al., 2023). Almost all populations developed a low to moderate level of resistance to pymetrozine and maintained moderate resistance to chlorpyrifos. A synergism assay using diethylmaleate, triphenyl phosphate, and piperonyl butoxide was performed on a field buprofezin-resistant population, and no significant synergistic effect was observed for the three inhibitors, implying that other mechanisms may be involved in resistance evolution in WBPHs IGR (Liu et al., 2023). Dinotefuran (85.6-97.7%), fenobucarb (82.9-100%), and carbosulfan (77.9-96.4%) demonstrated highest mortality rates among all samples in the study. Etofenprox EC (65.6–86.6%) and imidacloprid SC (69.0–84.8%) resulted in acceptable mortality rates. Both areas (western and southern) are far apart, but all six sites' WBPH populations showed similar patterns of mortality 96 hrs after treatment with each insecticide (Fig. 1). This is consistent with the previous study (Kang et al., 2022) which found that dinotefuran SG (100%), carbosulfan SC (89.7%), etofenprox EC (89.7%), and fenobucarb EC (100%) all exhibited high mortalities at 96 hrs except imidacloprid SC (96.6%). Two carbamates, fenobucarb and carbosulfan, have been applied to rice to control a wide range of sucking insects, BPHs, and SBPHs in the rice-producing countries including Vietnam and Cambodian (Park and Cho, 2020). Fenobucarb has been officially registered in South Korea to control small brown planthoppers (SBPHs), BPHs, and WBPHs.
Many neonicotinoids including, imidacloprid, dinotefuran, and thiamethoxam are selective agonists of nicotinic acetylcholine receptor in insects and have been used in the field to control sucking pests such as RPHs in most of rice producing countries (Bass et al., 2015; Wang et al., 2008). Imidacloprid, the first developed neonicotinoid insecticide, was introduced into China in the early 1990s, and some WBPH field populations developed low to moderate resistance to it between 2010–2013 (Zhang et al., 2014). While dinotefuran and etofenprox showed high mortality rate in this research, a previous study (Li et al., 2021) exhibited low to moderate resistance to dinotefuran, etofenprox, and imidacloprid. Another monitoring study (Liu et al., 2023) indicated that most field populations remained susceptible to dinotefuran before 2020, with the exception of a few populations that developed low resistance and reached low to moderate resistance by 2022. Between 2019 and 2022, the Yangan population resistance ratio increased significantly. This phenomenon could be possibly due to the frequent use of dinotefuran in this region, implying that the risk of increased WBPH resistance to dinotefuran has emerged in the field. A previous study, which ran from 2014 and 2022 showed that resistance to imidacloprid had increased significantly in all field populations over seven years. In 2016, all field populations were susceptible to imidacloprid, and resistance has gradually increased since then. The study indicated that an increasing proportion of monitored populations developed moderate imidacloprid resistance after 2019, reaching two-thirds in both 2020 and 2022 (Liu et al., 2023).
Monitoring data from this experiment showed that the three tested insecticides (dinotefuran, carbosulfan, and fenobucarb), were effective in controlling WBPHs, while the two insecticides (pymetrozine and buprofezin) were ineffective. In the case of etofenprox, the WBPHs from sites 2, 4, 5, and 6 were not effectively controlled. It demonstrated that the regular monitoring process can provide basic data for effectively using insecticides to control these planthoppers in rice paddies. Considering that the extensive use of a single pesticide can be the main cause of unexpected rapid population development of the pests (Li et al., 2021; Liu et al., 2023), monitoring data can serve as important indicators for more judicious and efficient management practice. Monitoring of the insecticide resistance will continue to be required to investigate the exact causes of a control failure, such as insecticide resistance development, improper application, and unexpected pest outbreak. However, given reports of migratory connectivity indicating that BPHs and WBPHs migrating into temperate East Asia (China, Japan, and South Korea) returns to their original locations through the wind in late summer and autumn (Gao et al., 2020), reports from other countries (particularly, China) must also be considered. We speculate that the periods when BPHs and WBPHs fly to South Korea and the use of the same insecticides in the same rice paddy areas, could pose significant problems. Therefore, different classes of insecticides for alternate spray should be officially reviewed, to take rapid action against RPHs in South Korea based on monitoring data as well as resistance reports from other possible immigration sources. We recommend that WBPHs enzyme activities should be determined for further confirmation of their resistance against these insecticides.
Acknowledgments
This research was carried out with the funding of “Research Program for Agricultural Science & Technology Development (Project No. RS-2020-RD008881), National Institute of Agricultural Science, Rural Development Administration, Republic of Korea.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
References
-
Abbott WS, 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18(2):265-267.
[https://doi.org/10.1093/jee/18.2.265a]

- Rural Development Adminitration, 2012. Asian Food and Agriculture Cooperation Initiative (AFACI), Asia migratory insects and viruses surveillance system (AMIVS). https://www.amivs.org/amivs/mainr.ea, (Accessed Feb. 15. 2024).
-
Ausborn J, Wolf H, Mader W, Kayser H, 2005. The insecticide pymetrozine selectively affects chordotonal mechanoreceptors. J. Exp. Biol. 208: 4451-4466.
[https://doi.org/10.1242/jeb.01917]

-
Bass C, Denholm I, Williamson MS, Nauen R, 2015. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 121: 78-87.
[https://doi.org/10.1016/j.pestbp.2015.04.004]

-
De Cock A, Degheele D, 1998. Buprofezin: A novel chitin synthesis inhibitor affecting specifically planthoppers, whiteflies and scale insects, in: Ishaaya, I., Degheele, D. (Eds.), Insecticides with Novel Modes of Action: Mechanisms and Application. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 74-91.
[https://doi.org/10.1007/978-3-662-03565-8_5]

-
Gao B, Hedlund J, Reynolds DR, Zhai B, Hu G, et al., 2020. The ‘migratory connectivity’ concept, and its applicability to insect migrants. Mov. Ecol. 8: 48.
[https://doi.org/10.1186/s40462-020-00235-5]

-
Huang R, Meng H, Wan H, Li J, Zhang X, 2022. Field evolution of insecticide resistance against Sogatella furcifera (Horváth) in Central China, 2011-2021. Agronomy 12(10): 2588.
[https://doi.org/10.3390/agronomy12102588]

-
Jianya S, Zhiwei W, Kai Z, Xiangrui T, Yanqiong Y, et al., 2013. Status of insecticide resistance of the whitebacked planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Fla. Entomol. 96: 948-956.
[https://doi.org/10.1653/024.096.0332]

-
Jin JX, Jin DC, Li WH, Cheng Y, Li FL, Ye ZC, 2017. Monitoring trends in insecticide resistance of field populations of Sogatella furcifera (Hemiptera: Delphacidae) in Guizhou province, China, 2012–2015. J. Econ. Entomol. 110(2): 64–650.
[https://doi.org/10.1093/jee/tox027]

-
Jeong IH, Jeon SW, Lee SK, Park B, Park SK, et al., 2017. Insecticide cross-resistance and developmental characteristics on the two rice varieties, ‘Chinnong’ and ‘Chuchung’, of the imidacloprid-resistanct brown planthopper. Korean J. Pestic. Sci. 21(4):381-388. (In Korean)
[https://doi.org/10.7585/kjps.2017.21.4.381]

-
Kang Y, Koo H-N, Kim H-K, Kim GH, 2022. Analysis of the feeding behavior and life table of Nilaparvata lugens and Sogatella furcifera (Hemiptera: Delphacidae) under sublethal concentrations of imidacloprid and sulfoxalfor. Insects 13(12): 1130.
[https://doi.org/10.3390/insects13121130]

-
Kaufmann L, Schqrmann F, Yialloursa M, Harrewun P, Kayser H, 2004. The serotonergic system is involved in feeding inhibition by pymetrozine; comparative studies on a locust (Locusta migratoria) and an aphid (Myzus persicae). Comp. Biochem. C. 138: 469-483.
[https://doi.org/10.1016/j.cca.2004.08.007]

-
Li H, Xu L, Wu W, Peng W, Lou Y, et al., 2023. Infestation by the piercing-sucking herbivore Nilaparvata lugens systemically triggers JA- and SA-dependent defense responses in rice. Biology 12(6): 820.
[https://doi.org/10.3390/biology12060820]

-
Li Z, Qin Y, Jin R, Zhang Y, Ren Z, et al., 2021. Insecticide resistance monitoring in field populations of the whitebacked planthopper Sogatella furcifera (Horváth) in China, 2019-2020. Insects 12(12): 1078.
[https://doi.org/10.3390/insects12121078]

-
Liu Y-T, Song X-Y, Zeng B, Zhang W-J, Chen X-Y, et al., 2023. The evolution of insecticide resistance in the white backed planthopper Sogatella furcifera (Horváth) of China in the period 2014–2022. Crop Prot. 172: 106312.
[https://doi.org/10.1016/j.cropro.2023.106312]

-
Maienfisch P, 2019. Selective feeding blockers: pymetrozine, flonicamid, and pyrifluquinazon, Modern Crop Protection Compounds, pp. 1501-1526.
[https://doi.org/10.1002/9783527699261.ch34]

-
Matsumura M, 2001. The current status of occurrence and forecasting system of rice planthoppers in Japan. J. Asia. Pac. Entomol. 4(2): 195-199.
[https://doi.org/10.1016/S1226-8615(08)60123-5]

-
Matsumura M, Sanada-Morimura S, Otuka A, Sonoda S, Thanh DV, et al., 2018. Insecticide susceptibilities of the two rice planthoppers Nilaparvata lugens and Sogatella furcifera in East Asia, the Red River Delta, and the Mekong Delta. Pest Manag. Sci. 74(2): 456-464.
[https://doi.org/10.1002/ps.4729]

-
Matsumura M, Takeuchi H, Satoh M, Sanada-Morimura S, Otuka A, et al., 2008. Species-specific insecticide resistance to imidacloprid and fipronil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in East and South-east Asia. Pest Manag. Sci. 64(11): 1115-1121.
[https://doi.org/10.1002/ps.1641]

-
Matsumura M, Sanada-Morimura S, Otuka A, Ohtsu R, Sakumoto S, et al., 2014. Insecticides in populations of two rice planthoppers, Nilaparvata lugens and Sogatella furcifera immigrating into Japan in the period 2005-2012. Pest Manag. Sci. 70(4): 615-622.
[https://doi.org/10.1002/ps.3590]

-
Mu X-C, Zhang W, Wang L-X, Zhang S, Zhang K, et al., 2016. Resistance monitoring and cross-resistance patterns of three rice planthoppers, Nilaparvata lugens, Sogatella furcifera and Laodelphax striatellus to dinotefuran in China. Pestic. Biochem. Phys. 134: 8-13.
[https://doi.org/10.1016/j.pestbp.2016.05.004]

-
Otuka A, Matsumura M, Watanabe T, 2009. The search for domestic migration of the white-backed planthopper, Sogatella furcifera (Horváth) (Homoptera: Delphacidae), in Japan. Appl. Entomol. Zool. 44(3): 379-386.
[https://doi.org/10.1303/aez.2009.379]

-
Park B, Cho JR, 2020. Survey on insecticides for controlling migratory rice planthoppers in major rice production countries of Southeast Asia. J. Korean Soc. Int. Agric. 32: 315-319. (In Korean)
[https://doi.org/10.12719/KSIA.2020.32.4.315]

-
Su J, Wang Z, Zhang K, Tian X, Yin Y, 2013. Status of insecticide resistance of the whitebacked planthopper, Sogatella furcifera (Hemiptera:Delphacidae). Fla. Entomol. 9(3): 948-956.
[https://doi.org/10.1653/024.096.0332]

-
Wang Y, Chen J, Zhu YC, Ma C, Huang Y, et al., 2008. Susceptibility to neonicotinoids and risk of resistance development in the brown planthopper, Nilaparvata lugens (Stal) (Homoptera: Delphacidae). Pest Manag. Sci. 64(12): 1278–1284.
[https://doi.org/10.1002/ps.1629]

-
Wu S-F, Zeng B, Zheng C, Mu X-C, Zhang Y, et al., 2018. The evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) of China in the period 2012–2016. Sci. Rep. 8: 4586.
[https://doi.org/10.1038/s41598-018-22906-5]

-
Yin Y, Li X, Chu D, Zhao X, Sathya K, et al., 2017. Extensive gene flow of white-backed planthopper in the Greater Mekong Subregion as revealed by microsatellite markers. Sci. Rep. 7: 15905.
[https://doi.org/10.1038/s41598-017-16164-0]

-
Zhang G, Wu Y, Li X-J, Hu G, Lu M-H, et al., 2016. Annual fluctuations of early immigrant populations of Sogatella furcifera (Hemiptera: Delphacidae) in Jiangxi Province, China. J. Econ. Entomol. 109(4): 1636-1645.
[https://doi.org/10.1093/jee/tow136]

-
Zhang K, Zhang W, Zhang S, Wu SF, Ban LF, et al., 2014. Susceptibility of Sogatella furcifera and Laodelphax striatellus (Hemipter: Delphacidae) to six insecticides in China. J. Econ. Entomol. 107(5): 1916-1922.
[https://doi.org/10.1603/EC14156]

-
Zhou GH, Wen JJ, Cai DJ, Li P, Xu DL, et al., 2008. Southern rice black-streak dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chinese Sci. Bull. 53: 3677-3685.
[https://doi.org/10.1007/s11434-008-0467-2]

Leesun Kim, Crop Protection Division, National Institute of Agricultural Sciences, Rural Development Administration, Postdoctoral researcher, https://orcid.org/0000-0003-1174-8877
Wonyoung Kim, Crop Protection Division, National Institute of Agricultural Sciences, Rural Development Administration, Researcher
Bueyong Park, Crop Protection Division, National Institute of Agricultural Sciences, Rural Development Administration, Senior researcher
Hong-Hyun Park, Crop Protection Division, National Institute of Agricultural Sciences, Rural Development Administration, Senior researcher
Meeja Seo, Crop Protection Division, National Institute of Agricultural Sciences, Rural Development Administration, Senior researcher
In-hong Jeong, Crop Protection Division, National Institute of Agricultural Sciences, Rural Development Administration, Senior researcher, https://orcid.org/0000-0002-4625-2268
Methodology & Writing-Original Draft Preparation; Leesun Kim, Experimentation; Wonyoung Kim, Investigation; Bueyong Park, Data Analysis; Hong-Hyun Park, Writing-Review & Editing, Data Analysis; Meeja Seo, Conceptualization & Supervising; In-Hong Jeong