PCR Based Assays for the Specific Detection of Colletotrichum species Causing Apple Bitter Rot in Korea
Abstract
Colletotrichum species of the C. gloeosporioides species complex are the major pathogens of bitter rot of apples in Korea. To ensure early and quick detection of these species, species-specific primers based on the single nucleotide polymorphisms (SNPs) in the nucleotide sequences of the β-tubulin gene were designed and used in PCR assays. Primers were designed by making the SNP the 3‘ end and introducing a single nucleotide artificial mismatch at the penultimate base to the SNP. Primer pairs Cae-Bt462F/CgSc-Bt691R, Cfr-Bt492F/CgSc-Bt691R, Cgl-591F/CgSc-Bt691R and Csi-Bt567F/CgSc-Bt691R successfully amplified specific PCR fragments from C. aenigma, C. fructicola, C. gloeosporioides and C. siamense respectively in pure cultures and inoculated apple fruits. Each primer pair only amplified fragments from their respective species. The sensitivity assay to determine the lowest detectable DNA concentration of the primers showed that the primers could detect as low as 10 to 100 pg of purified DNA. A duplex PCR assay was conducted with primer pairs Cfr-Bt492F/CgSc-Bt691R and Csi-Bt567F/CgSc-Bt691R for the detection of C. fructicola and C. siamense, the two most isolated species from bitter rot infected apple in Korea. The primers could specifically detect each species from one reaction. This PCR-based protocol could be used for quick detection of Colletotrichum gloeosporioides species complex causing apple bitter rot in Korea and could save the cost of bitter rot diagnosis.
Keywords:
Apple bitter rot, PCR, Colletotrichum species, Species-specific primer, Duplex PCRIntroduction
Colletotrichum species within the C. gloeosporioides and C. acutatum species complexes have been reported to cause bitter rot of apples in Korea. Among these species, C. fructicola, C. siamense, C. gloeosporioides and C. aenigma, within the C. gloeosporioides species complex and C. fiorinieae and C. nymphaeae, belonging to the C. acutatum species complex have over the years been isolated from bitter rot-infected apples in Korea (Kim et al., 2018; Lee et al., 2007; Lee et al., 2021; Oo et al., 2018; Park et al., 2018). Within the last three years, most of the isolates from bitter rot-infected apples have been C. fructicola and C. siamense (Kim et al., 2023). Interestingly, the two species may differ in their sensitivities to the commonly used fungicides, particularly those of the benzimidazoles and quinone outside inhibitors (Abdullahi et al., 2023). Therefore, identification of these species is necessary to understand disease epidemiology and develop strategies to control the disease successfully (Cai et al., 2009). Current identification of these species relies mostly on the use of some genetic markers, such as glyceraldehyde 3-phosphate dehydrogenase (GAPDH), beta-tubulin (TUB2), actin (ACT), calmodulin (CAL), chitin synthase (CHS-1), histone-3 (His3) and internal transcribed spacer (ITS) region (Liu et al., 2012; Vieira et al., 2020). Owing to their high sequence similarity, one marker is insufficient and at least three of these markers offer sufficient information for discrimination of these species (Vieira et al., 2020). The high cost of sequencing these genes and the time spent in preparing samples pose challenges to the quick identification of these species, particularly for small laboratories.
Species-specific polymerase chain reaction (PCR) based assays are commonly used for the identification and detection of fungal species, offering less time and saving cost of gene sequencing. Species-specific primer designs to target unique regions of the target species with high sequence variability have been used for the identification of some fungal organisms (Ismadi et al., 2023; Luo and Mitchell, 2002; Yamashita et al., 2018). However, higher consensus regions among the species within C. gloeosporioides species complex in the commonly used genetic markers have made this primer design difficult. ITS region for example is only ideal for designing specific primers to detect fungi belonging to different genera, due to its variability among these genera but highly limited in specific primer design for fungi within a species complex (White et al., 1990). Though GAPDH, His3, and TUB2 have been adjudged three of the best markers for discriminating members of this complex (Vieira et al., 2020), most of the sequence variability found among these species in GAPDH and His3 including ACT are contained in the intron region of the gene. TUB2 contains more variable sequences among these species in the exon region of the gene but are mainly single nucleotide polymorphisms (SNPs).
Several studies have reported the use of SNPs in species-specific primer design for the identification of some fungal species and other microorganisms (Kalendar et al., 2022; Liu et al. 2012; Nawaz et al., 2018). The 3’ end of the primer (forward and/or reverse) is usually targeted to coincide with the SNP. However, a single base mismatch at the very 3’ end still allows amplification, though at a reduced efficiency for some cases. The consensus is that a two-base mismatch at the 3’ end generally prevents amplification (Ye et al., 2012). It is difficult to find a two-base mismatch in the nucleotide sequences of Colletotrichum species within the C. gloeosporioides species complex infecting apples in the commonly used genetic markers. With the limitation of using only SNPs for designing species-specific primers, and the need for quick detection of these species directly from bitter rot-infected apple fruits, this study was aimed at developing an SNP-based species-specific PCR assays for the detection of Colletotrichum species within the C. gloeosporioides species complex causing bitter rot of apples in Korea.
Materials and Methods
DNA extraction
Twenty-four isolates of Colletotrichum species were used in this study (Table 1). These included four isolates each of C. aenigma, C. fructicola, C. gloeosporioides, and C. siamense, all belonging to C. gloeosporioides species complex and four isolates each of, which are C. fioriniae and C. nymphaeae of the C. acutatum species complex. These species were isolated from bitter rot-infected apples in 2015, 2016, 2021, 2022, and 2023. These isolates, previously stored at -70oC in 20% glycerol were revived on potato dextrose agar (PDA; DifcoTM, Becton, Dickinson and Company, Sparks, MD21152 USA). After incubation in the dark at 25℃ on PDA, the mycelia were harvested and transferred into a 2.0 mL microcentrifuge tube, freeze-dried for 24 hours, and used for DNA extraction. Total DNA from each isolate was extracted using a Plant SV mini extraction kit (GeneAll Biotechnology, Korea) following the manufacturer’s instructions.
Species-specific primer design
The full length sequences of the TUB2 region of these isolates were amplified using three primer pairs (Table 2). The amplicons were purified and sequenced using Sanger sequencing. The obtained sequences of these isolates together with other reference sequences of the Colletotrichum species were analysed using the CLUSTAL W program of MEGA 11 software. From the aligned sequences, SNPs for each species were identified. Species-specific forward primers were designed with the SNP at the 3’ end while altering the penultimate base of the primer. For instance, in designing a specific primer for C. aenigma, an SNP with thymine (T) was identified at the 480th mRNA nucleotide position of the TUB2, whereas all other Colletotrichum species carry a cytosine (C) at the same position. The 479th base which was also a C was then substituted with another T to create a mismatch at the penultimate base end of the primer. This procedure creates only one mismatch from the target template, but two mismatches at the 3‘-end of the non-target template, thus avoiding possible amplification of the latter. A 19-base primer was then designed from the 480th base backward for detection of C. aenigma. This procedure was followed for other Colletotrichum species. A common reverse primer from the conserved region of the gene was designed for all the species. The designed primers were analysed for GC content, Tm, as well as hairpin, self-dimer, and heterodimer formation using integrated DNA technologies online oligo analyser tools (https://sg.idtdna.com/calc/analyzer). SNPs that would result in a primer with more than 70% GC content and more than three ‘G’s or ‘C’s in the 3’-end due to the creation of a C or G mismatch were avoided. This is because of the possibility of potential annealing at multiple sites. Where this was not possible as in the case of C. fructicola, another mismatch with a T was introduced towards the 5’ end of the primer.
Validating primers for specificity and sensitivity
DNA templates of four representative isolates of each species were selected to test the specificity of the primers. For each primer pair, PCR was carried out in a 20 µL reaction volume containing 4 µL master mix (EzPCR 5X PCR master mix, Elpis Biotech.), 1 µL each of the forward and reverse primers, 2 µL gDNA and 12 µL sterile distilled water (SDW). To determine the lowest detectable DNA template concentration of the species-specific primers, DNA templates of the species were serially diluted from 100 ng/µL to 10 pg/µL, and 1µL was used as a template for the PCR. PCR conditions included initial denaturation at 95℃ for 2 mins, followed by 30 cycles each involving denaturation at 94oC for 30 sec, annealing at 55oC for 30 sec, extension at 72oC for 1 min, and then final extension at 72oC for 5 mins. The PCR products were separated by electrophoresis in 1% agarose gel in Tris-Borate (TBE) buffer for 40 min at 100 volts and viewed using a transilluminator.
Detection of Colletotrichum species from inoculated apple fruits
Eight representative isolates comprising C. aenigma, C. fructicola, C. gloeosporioides, and C. siamense were selected for inoculation into apple fruits. Healthy apple fruits were collected from a commercial orchard, washed with tap water, and disinfected in 1% sodium hypochlorite (NaOCl) for three minutes. The disinfected apples were washed with sterilized water three times, dried in a laminar flow hood, and then placed on a sterilized plastic saucer in a sterilized plastic box with wet tissue. The apples were inoculated with 10 µL each of 104 spore/mL concentration of the isolates. Control apple fruit received SDW. Three replications were maintained for each isolate as well as the control. Plastic boxes containing inoculated apple fruits and the control placed on moist paper towels were sealed and incubated at 25oC for 72 hours in the dark to allow for a post-inoculation period of high humidity. Thereafter seals were removed and boxes were opened for air and tissue paper wetting. Ten days after inoculation, infected tissues were excised for DNA extraction using the method described earlier. The species-specific PCR assay and agarose gel electrophoresis were conducted as described above.
Duplex PCR assay
One isolate each of C. fructicola and C. siamense was selected for a multiplex PCR assay in a 20 µL PCR mixture containing 4 µL master mix (EzPCR 5X PCR master mix, Elpis Biotech.), 0.5 µL (0.25 pmol/µL) each of the forward primers, 1 µL (0.5 pmol/µL) of the reverse primer, 1 µL gDNA each of the isolate and 12 µL sterile distilled water (SDW). PCR conditions and agarose gel electrophoresis are described above.
RESULTS
Species-specific primer design
The sequence similarity in the beta-tubulin gene of C. aenigma, C. fructicola, C. gloeosporioides, and C. siamense associated with apple bitter rot was analysed. A comparison of the sequences indicated that the TUB2 genes of these species are highly conserved with only synonymous SNPs distinguishing among the species, which were either by transition (>90%) or by transversion (<10%). Using the SNPs with the method described above, four species-specific forward primers and a common reverse primer were designed to amplify the sequences of four Colletotrichum species causing bitter rot of apples (Table 3).
Primer specificity
The specificity of the primers was tested using genomic DNA extracted from the mycelia of the isolates (Table 2). The four primer pairs for members of the C. gloeosporioides species complex could amplify a fragment from the four species respectively. Primer pair Cae-Bt462F/CgSc-Bt691R produced 230 bp amplicon from C. aenigma isolates 16MPDY5, 16MPDY19, JlJaCG22-2-1, and YC2RUB3 and none from other isolates. The primer pair Cfr-Bt492F/CgSc-Bt691R produced 200 bp amplicon from C. fructicola isolates 16MPYD10, 16MPYD11, DO35, and DO39, but none from other isolates. 16MPDY1, 16MPDY4, 16MPDY16, and GgYcCG23-3-1 isolates of C. gloeosporioides were amplified by the primer pair Cgl-Bt591F/CgSc-Bt691R, producing 101 bp fragment but none of the isolates from other species was amplified. Primer pair Csi-Bt567F/CgSc-Bt691R produced 125 bp amplicon from the C. siamense isolates 16MPYD19, 16MPDY2, 16MPDY3, and 16MPBH1, but none from the other isolates (Fig. 1). The primers were also used to amplify C. fioriniae and C. nymphaeae of the C. acutatum species complex isolated from apple but no band was produced (data not shown).
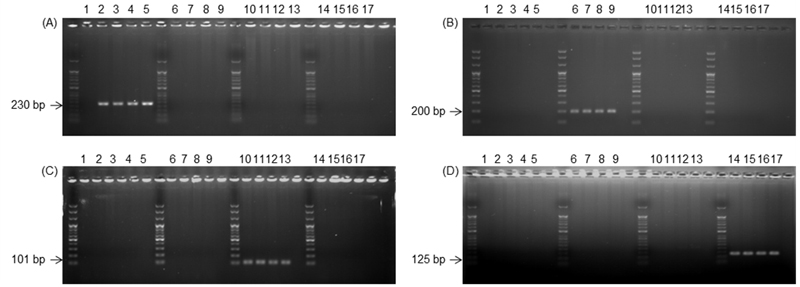
Gel electrophoresis showing the specific bands of the fragment of β-tubulin gene produced by each primer. (A); Colletotrichum aenigma specific primers Cae-Bt462F/CgSc-Bt691R, (B); C. fructicola specific primers Cfr-Bt492F/CgSc-Bt691R, (C); C. gloeosporioides specific primers Cgl-591F/CgSc-Bt691R, (D); C. siamense specific primers Csi-Bt567F/CgSc-Bt691R. Four isolates of each of the four species of Colletotrichum were selected and used in the experiment. 1; negative control, 2-5; C. aenigma 16MPDY5, 16MPDY19, JlJaCG22-2-1, and YC2RUB3, 6-9; C. fructicola 16MPYD10, 16MPYD11, DO35, and DO39, 10-13; C. gloeosporioides 16MPDY1, 16MPDY4, 16MPDY16, and GgYcCG23-3-1, 14-17; C. siamense 16MPYD19, 16MPDY2, 16MPDY3, and 16MPBH1.
Primer sensitivity
The sensitivities of the primers were tested by 10-fold dilution of template DNA of 100 ng/µL, 10 ng/µL, 1 ng/µL, 100 pg/µL and 10 pg/µL. Primer pair Cae-Bt462F/CgSc-Bt691R could detect DNA concentration as low as 10 pg/µL, while the lowest detectable DNA concentration by the three other primers was 100 pg/µL each (Fig. 2).
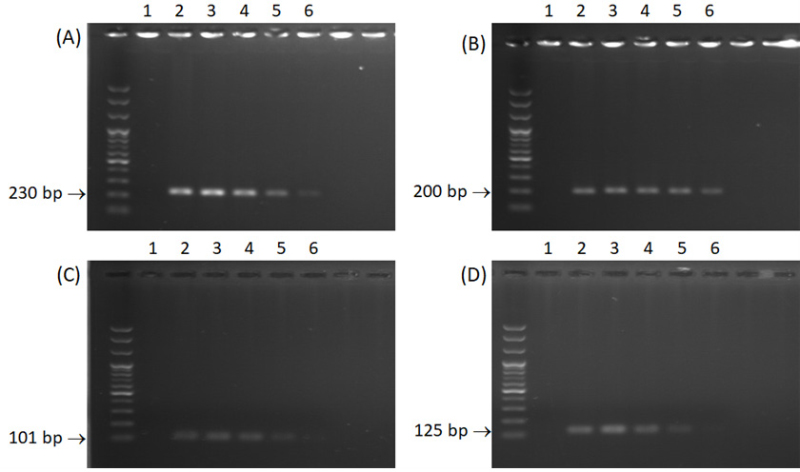
Gel electrophoresis showing the specific bands of the fragment of β-tubulin gene produced by each primer for each DNA concentration. (A); Colletotrichum aenigma specific primers Cae-Bt462F/CgSc-Bt691R, (B); C. fructicola specific primers Cfr-Bt492F/CgSc-Bt691R, (C); C. gloeosporioides specific primers Cgl-591F/CgSc-Bt691R, (D); C. siamense specific primers Csi-Bt567F/CgSc-Bt691R. One isolate per Colletotrichum species was used in this experiment, C. aenigma 16MPDY5, C. fructicola 16MPYD10, C. gloeosporioides 16MPDY1, and C. siamense 16MPYD19. The numbers indicate the concentration of DNA used in this experiment. As follows: 1; negative control, 2; 100 ng/μL, 3; 10 ng/μL, 4; 1 ng/μL, 5; 100 pg/μL, and 6; 10 pg/μL.
Detection of the Colletotrichum species from inoculated apple fruits
DNA from bitter rot lesions resulting from inoculated apple fruits was extracted and a PCR assay was carried out to determine whether the primers could detect Colletotrichum species in artificially inoculated apple fruits. The results showed that the primer pairs of Cae-Bt462F/CgSc-Bt691R, Cfr-Bt492F/CgSc-Bt691R, Cgl-Bt591F/CgSc-Bt691R, and Csi-Bt567F/CgSc-Bt691R yielded the expected single band of 230 bp, 200 bp, 101 bp, and 125 bp respectively (Fig. 3).
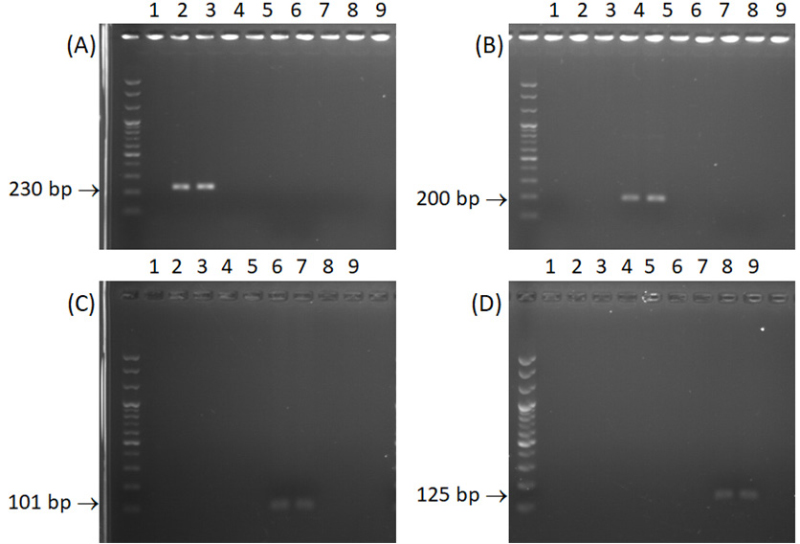
Gel electrophoresis of PCR with each species-specific primer using gDNA extracted from diseased apples as a template. In this experiment, 4 primer sets as Cae-Bt462F/CgSc-Bt691R (A), Cfr-Bt492F/CgSc-Bt691R (B), Cgl-591F/CgSc-Bt691R (C) and Csi-Bt567F/CgSc-Bt691R (D) were used to detect Colletotrichum spp. included into C. gloeosporioides species complex. For the inoculation of Colletotrichum spp. to apple fruits, conidial density was adjusted to 1 × 106 conidia/mL. After inoculation, apple fruits were kept at 25oC for 3 days in humidity chamber. Thereafter, gDNA was extracted from the initial lesions 7 days after inoculation. Numbers indicate the isolates of Colletotrichum species used in the experiment, where two isolates of each Colletotrichum species were used, as follows. 1; negative control, 2; Colletotrichum aenigma 16MPDY5, 3; C. aenigma JlJaCG22-2-1, 4; C. fructicola 16MPYD10, 5; C. fructicola DO39, 6; C. gloeosporioides 16MPDY1, 7; C. gloeosporioides GgYcCG23-3-1, 8; C. siamense 16MPYD19, and 9; C. siamense 16MPDY2.
Duplex PCR Assay
A duplex PCR assay with all four primer pairs could not produce distinct bands because of the closeness of the band sizes, 101bp/125bp and 200bp/230bp for C. gloeosporioides/C. siamense and C. fructicola/C. aenigma respectively. A duplex PCR assay involving primer pairs Cfr-Bt492F/CgSc-Bt691R and Csi-Bt567F/CgSc-Bt691R successfully detected C. fructicola and C. siamense from one reaction, producing 200 bp and 125 bp respectively (Fig. 4).
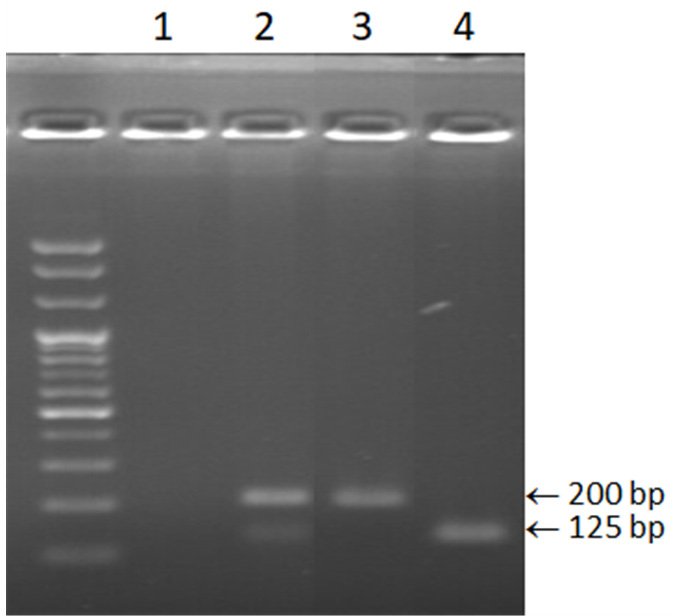
Gel electrophoresis showing duplex PCR for the detection of Colletotrichun fructicola and C. siamense amplified with primer pairs Cfr-Bt492F/CgSc-Bt691R and Csi-Bt567F/CgSc-Bt691R, respectively. 1; negative control, 2; C. fructicola 16MPYD10 and C. siamense 16MPDY2, 3; C. fructicola 16MPYD10, 4; C. siamense 16MPDY2.
Discussion
Apple bitter rot caused by Colletotrichum species is one of the most devastating fruit diseases in Korea. At least six Colletotrichum species belonging to C. gloeosporioides and C. acutatum complexes have been identified over the years to be associated with this disease (Kim et al., 2018; Lee et al., 2007; Oo et al., 2018; Park et al., 2018). However, the current status of the disease in the country shows that members of the C. gloeosporioides complex are the major pathogens of apple bitter rot (Abdullahi et al., 2023). Rapid identification of these species is necessary for epidemiology and management strategies. In this study, we designed single nucleotide polymorphism (SNP) based primers for the specific detection of these species from pure culture and disease apple fruits. The use of SNP for species-specific primer design for fungal species identification has been demonstrated in numerous studies, particularly for species with high sequence similarity in their genome (Liu et al., 2012; Nawaz et al., 2018; Xu et al., 2016). The most common SNP-based primer design places the SNP at the 3’ end of the primer (Liu et al., 2012), though there is a report of the SNP at the 5’ end of the forward primer (Nawaz et al., 2018). The major challenge of the SNP-based species-specific primer design is the possibility of amplification of the non-targets but a two-base mismatch at the 3’ end generally prevents amplification according to Ye et al. (2012). Xu et al. (2016) reported that introducing mismatches at the 2nd, 3rd, or 4th base from the SNP increases the specificity of the primers but with varying degrees and concluded that mismatches at the 3rd base from the SNP were the most efficient in discriminating the two lines of the Brassica oleracea. Attempts to design SNP-based primers with mismatches specified in that study could not produce the desired results. Here we introduced a mismatch of the same base as the SNP of the target at the 1st base (penultimate base) from the SNP, based on the nucleotide sequence of the β-tubulin gene. Using this method, species-specific primers designed with thymine as the SNP of the target had the highest efficiency and were more specific than those with cytosine or guanine. Those with a double G or C at the 3’ end, as in the case of the forward primer for detection of C. fructicola produced multiple bands (data not shown), which was probably due to high G/C content and/or more than three ‘G’s or ‘C’s in the 3’-end as a result of creation of a C or G mismatch (Javed and Ebertz, 2022). This was resolved by the creation of another thymine mismatch towards the 5’ end (16th base from SNP) of the primer.
McHenry and Acimovic (2024) reported species-specific real-time PCR assays for the detection of Colletotrichum species causing bitter rot of apples using primers designed from some gene regions specific for each species, but the study did not report direct detections from diseased apple fruits. The four primers designed in this study could detect each species from pure culture and diseased apple fruits, enhancing quicker detection of each species. The primers were also tested for other Colletotrichum species belonging to the C. acutatum species complex and none of these species could be detected by these primers. The sensitivity of a primer is important for early diagnosis of disease plants (Xu et al., 2016). Though the primers could detect as low as 100 pg DNA of pure culture of their respective species, the multiplex PCR assay involving the four primer sets could not successfully discriminate the four species. However, C. fructicola and C. siamense, the two most isolated species from bitter rot in Korea were successfully discriminated by a duplex PCR. The response of these two species to the commonly used fungicides differ markedly (Abdullahi et al., 2023), thus, quick detection could improve the fungicide management strategy of apple bitter rot in Korea. Also, this PCR protocol is much easier than the morphological method and more cost-effective than gene sequencing. In addition, the unique primer design approach described in this study could also be used to design species specific primers for other fungal species.
Acknowledgments
This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ016906). Rural Development Administration, Republic of Korea
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Abdullahi A, Nam YJ, Kim HT, 2023. Distribution and fungicide sensitivity of Colletotrichum species causing bitter rot of apples in Korea. 2023 KSPP Fall Meeting and International Conference, Jeju, Korea. 17-20 Oct. p. 134.
- Cai L, Hyde KD, Taylor PWJ, Weir BS, Waller JM, et al., 2009. A polyphasic approach for studying Colletotrichum. Fungal Divers. 39:183–204.
-
Carbone I, Kohn LMA, 1999. Method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3):553–556.
[https://doi.org/10.1080/00275514.1999.12061051]

- Ismadi YKM, Mohamad S, Harun A, 2023. Species-specific PCR primers for simultaneous detection of Aspergillus fumigatus, Aspergillus terreus, Candida albicans and Candida glabrata in invasive fungal infections. Malays. J. Pathol. 45(3):397–403.
- Javed K, Ebertz A, 2022. Primer design guide-the top 5 factors to consider for optimum performance. Eurofins Genomics. https://the-dna-universe.com/2022/09/05/primer-design-guide-the-top-5-factors-to-consider-for-optimum-performance/, (Accessed Sep. 22. 2022).
-
Kalendar R, Shustov AV, Akhmetollayev I, Kairov U, 2022. Designing allele-specific competitive extension PCR-based assays for high-throughput genotyping and gene characterization. Front. Mol. Biosci. 9:773956.
[https://doi.org/10.3389/fmolb.2022.773956]

-
Kim C, Hassan O, Lee D, Chang T, 2018. First report of bitter rot of apple caused by Colletotrichum fructicola in Korea. Plant Dis. 102(12):2653-2653.
[https://doi.org/10.1094/PDIS-05-18-0717-PDN]

-
Kim J, Kim HT, Jeon Y, 2023. Research to fungicide sensitivity of Colletotrichum spp. isolated from apple fruits in Cheongsong, Korea. Res. Plant Dis. 29(2):145-157. (in Korean)
[https://doi.org/10.5423/RPD.2023.29.2.145]

-
Lee DH, Kim DH, Jeon YA, Uhm JY, Hong SB, 2007. Molecular and cultural characterization of Colletotrichum spp. causing bitter rot disease of apple in Korea. Plant Pathol. J. 23(2):37–44.
[https://doi.org/10.5423/PPJ.2007.23.2.037]

-
Lee SY, Ten LN, Ryu JJ, Kang IK, Jung HY, 2021. Colletotrichum aenigma associated with apple bitter rot on newly bred cv. RubyS Apple. Res. Plant Dis. 27(2):70-75.
[https://doi.org/10.5423/RPD.2021.27.2.70]

-
Liu J, Huang S, Sun M, Liu S, Liu Y, et al., 2012. An improved allele-specific PCR primer design method for SNP marker analysis and its application. Plant Methods 8:34.
[https://doi.org/10.1186/1746-4811-8-34]

-
Luo G, Mitchell TG, 2002. Rapid identification of pathogenic fungi directly from cultures by using multiplex PCR. J. Clin. Microbiol. 40(8):2860-2865.
[https://doi.org/10.1128/JCM.40.8.2860-2865.2002]

-
McHenry, D. J. and Acimovic´, S. G. 2024. New Species-Specific Real-Time PCR Assays for Colletotrichum Species Causing Bitter Rot of Apple. Microorganisms, 12:878.
[https://doi.org/10.3390/microorganisms12050878]

-
Nawaz HH, Anam U, Rajaofera MJN, He Q, Liu W, et al., 2018. Development of SNP-based markers to identify Colletotrichum gossypii in upland cotton. Plant Dis. 102(7):1426-1433.
[https://doi.org/10.1094/PDIS-10-17-1672-RE]

-
Oo MM, Yoon HY, Jang HA, Oh SK, 2018. Identification and characterization of Colletotrichum species associated with bitter rot disease of apple in South Korea. Plant Pathol. J. 34(6):480-489.
[https://doi.org/10.5423/PPJ.FT.10.2018.0201]

-
Park MS, Kim BR, Park I, Hahm SS, 2018. First report of two Colletotrichum species associated with bitter rot on apple fruit in Korea – C. fructicola and C. siamense. Mycobiology 46(2):154–158.
[https://doi.org/10.1080/12298093.2018.1478220]

-
Vieira WADS, Bezerra PA, da Silva AC, Velosoa JS, Câmara MPS, et al., 2020. Optimal markers for the identification of Colletotrichum species. Mol. Phylogenet. Evol. 143:106694.
[https://doi.org/10.1016/j.ympev.2019.106694]

-
White TJ, Bruns TD, Lee SB, Taylor JW, 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. pp. 315-322. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (Eds.). PCR Protocols: a guide to methods and applications, Academic Press, New York, USA.
[https://doi.org/10.1016/B978-0-12-372180-8.50042-1]

-
Woudenberg JHC, Aveskamp MM, Gruyter J, de Spiers AG, Crous PW, 2009. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 22:56-62.
[https://doi.org/10.3767/003158509X427808]

-
Xu C, Zhang H, Chi F, Ji Z, Dong Q, et al., 2016. Species-specific PCR-based assays for identification and detection of Botryophaeriaceae species causing stem blight on blueberry in China. J. Integr. Agric. 15(3):573-579.
[https://doi.org/10.1016/S2095-3119(15)61177-7]

-
Yamashita S, Nakagawa H, Sakaguchi T, Arima TH, Kikoku Y, 2018. Design of a species-specific PCR method for the detection of the heat-resistant fungi Talaromyces macrosporus and Talaromyces trachyspermus. Lett. Appl. Microbiol. 66(1):86-92.
[https://doi.org/10.1111/lam.12818]

-
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, et al., 2012. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13:134 http://www.biomedcentral.com/1471-2105/13/134, .
[https://doi.org/10.1186/1471-2105-13-134]

Abdulkareem Abdullahi, Department of Plant Medicine, College of Agriculture, Life and Environment Science, Chungbuk National University, PhD student, Research investigation, development of allele-specific PCR, data analysis and first draft preparation. https://orcid.org/0000-0002-3841-2356
Heung Tae Kim, Department of Plant Medicine, College of Agriculture, Life and Environment Science, Chungbuk National University, Professor, Establishment of experimental plan and methodology proposal, writing original paper and editing. https://orcid.org/0000-0001-7132-0587

