Influence of Soil Types and Organic Amendment During Persistence, Mobility, and Distribution of Phorate and Terbufos in Soils
Abstract
The persistence, mobility and distribution patterns of phorate and terbufos in soil was not well explored. As a result, this study is designed to evaluate the residual uncertainty and leaching potential of phorate and terbufos in packed soil columns. The leaching and distribution behavior of applied pesticides will indicate the soil-pesticide interaction and groundwater contamination through soil column leaching. In this study, a comparative soil column study is conducted following the OECD guidelines for leaching soil column test for exploring the distribution, and mobility of phorate and terbufos including their metabolic transformation into soil column. Briefly, the packed soil column (5 cm diameter, 50 cm height) were filled up to 30 cm, and oversaturated by capillary waters. After leaching test, the leachate and soil sections (each section for 10 cm depth) were analyzed for parent pesticides and transformed metabolites. According to GUS index, both phorate and terbufos considered immobile pesticide (no residue is detected in the leachate) with low to extremely low leachability. Besides, the major pesticides and metabolite concentration (˃90% recovered) was found in the upper layer soil (0-10 cm) followed by subsoils. Among the transformed metabolites, sulfone and sulfoxide were the predominant for both pesticides. The degradation of phorate and terbufos was controlled by soil moisture, organic matter, and soil texture, respectively. In summary, the persistence, distribution and metabolic transformation of phorate and terbufos is limited within the vadose zone soil (0-10 cm) and no parent pesticides were detected in leachate due to slow leaching potential and immobility.
Keywords:
Phorate, Terbufos, Pesticide mobility, Persistence, Metabolite, Soil columnIntroduction
In global plant protection, pesticides are applied prudently through modern agriculture following good agricultural practices (GAP), but the incidental overuse of pesticide may results in residual uncertainty in the applied sites (Varjani et al. 2019). However, soil is considered a universal sink for pesticides, heavy metals and related organic pollutants (Sarker et al. 2020; Sarker et al. 2021a; Wang et al. 2020). In general, the environmental fate of pesticides and dissipation behavior is largely dependent on the soil-, pesticide-, and climatic factors (Gaonkar et al., 2019). Although, dissipation and distribution of different pesticides using various soil texture, organic matter and moisture contents were meticulously explored, still research gap regarding residual uncertainty exists (Katagi, 2013; Holten et al., 2019). In particular, phorate and terbufos are two non-ionic organophosphate pesticides used in controlling various sucking insect pest on cereals, corns, and vegetables. Although, phorate is moderately persistent and terbufos showing non-persistency in the field, the oxidative transformation of parent pesticides into sulfoxide and sulfone was evident with higher toxicity than parent pesticides (Jariyal et al., 2018). In addition, the persistence and leaching mobility of transformed metabolite (e.g, sulfoxide and sulfone) was found to be higher in the soil than parent pesticides (e.g., phorate and terbufos).
On the other hand, the contamination of groundwater through leaching of organic contaminants including pesticides is a critical concern for modern agriculture (Pérez-Lucas et al., 2020). At large, the judicious application of pesticides are permitted for the intensive agricultural practices following the good agricultural practice (GAP). However, the mobile pesticide can easily go through with soil pores (including macropores and micropores), and the leaching water flow may resulting in potential groundwater contamination (Shi et al., 2022). In contrast, the non-polar pesticides are considered moderately mobile or immobile pesticides, which can be deposited in the soil layers or partially leached into the groundwater (Katagi, 2013). Thus, solubility of pesticides is correlated with pesticide adsorption and leaching through water into deeper soil layer or into groundwater.
The key governing factors including pesticide properties, soil characteristics, interaction of pesticides with soil organic matter, moisture contents and soil microbes are all affecting the dissipation and leaching of pesticides. In particular, the sorption potential of pesticide has a negative correlation with pesticide leaching (Magga et al., 2008; Katagi, 2013; Hall et al., 2015; Rasool et al., 2022). Thus, highly adsorbed pesticides onto the soils have little to no impact on groundwater contamination. In addition, the level of organic matter (OM) have a significant impact on the fate of applied pesticides including adsorption, degradation, and leaching through soil water (Song et al., 2008; Marín-Benito et al., 2013; Sarkar and Mukherjee, 2021; Cara et al., 2022; Fouad, 2023). The leaching of pesticide is also dependent on the soil types with diverse physicochemical properties. Thus, meticulous studies are designed to explore the triggering factors during pesticide movements, leaching and degradation in the soil profile (Khorram et al., 2015; Barba et al., 2020; Cueff et al., 2020).
The typical leaching movement and distribution of pesticides is evaluated through soil column experiments (e.g., core soil column, and packed soil column) (Bindumol and Harilal, 2017; Holten et al., 2019). Besides, the lysimeter experiment is regularly performed in the real field conditions to mimic the real field situations. The benefit of lysimeter over packed soil column is the real field condition data can be obtained, but it is difficult to setting and managing lysimeter as compared to laboratory soil columns (Katagi, 2013). Besides, the classical thin layer chromatography (TLC) is employed for pesticide leaching behavior assessment (Mendes et al., 2019).
The initial process of pesticide breakdown is hydrolysis followed by plant uptake, soil deposition, adsorption-desorption, chemical degradation, biodegradation, volatilization, and leaching loss (Varjani et al., 2019; Kumari et al., 2020; Sarker et al., 2021b). The degradation and mobility of pesticides in the soils governed by the various soil- and pesticide-factors including soil moisture, organic matter, pH, pesticide solubility, and temperature (Rasool et al., 2022). However, the transformation of parent pesticides into toxic metabolites is considered as a critical threat for safety guidelines of applied pesticides (Carpio et al., 2021). According to previous report, the sorption, degradation, and leaching of ionic pesticides was controlled by soil pH, while negligible effect was evident for non-ionic pesticides (Manna and Singh, 2019; Marín-Benito et al., 2021; Cara et al., 2022). Besides, the leaching behavior and distribution of non-ionic pesticides are mostly governed by soil organic matter and textural class of soils.
In earlier studies, the role of organic amendment (organic manures and biochar) during leaching and mobility of pesticides in soil column was evaluated (Mendes et al., 2019; Pérez-Lucas et al., 2021). Although, pesticide mobility, persistence and leaching was explored by previously published research but there is no detailed study reported for distribution of phorate, and terbufos into soil layers including their mobility and leaching potential for groundwater contamination. As a result, this study is the pioneering approach for exploration of environmental fate including persistence, distribution, metabolic transformation and mobility of two non-ionic pesticides under various soil factors. The specific objectives of this study are: i) to assess the distribution of phorate, terbufos and their metabolites transformed into packed soil column following the OECD guideline, ii) to evaluate the persistence, degradation and transformation of parent phorate and terbufos into metabolites, iii) to study the mobility and leaching potential of phorate and terbufos under various soil factors such as soil texture, organic amendments, and iv) to compare the sorption potential of pesticides with leaching behavior during soil column investigations.
Methods and materials
Pesticides and chemicals
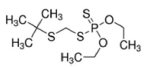
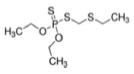
The analytical standard of phorate and terbufos having purity ≥95.0% were purchased from Sigma-Aldrich (Marketed by Merck KGaA, Darmstadt, Germany). The preparation of stock solution and working solution was performed using acetonitrile as organic solvent following the standard protocol. The stock solution of pesticides were kept at –20oC until further use. The other HPLC-grade chemicals (e.g., methanol, acetonitrile, water, ammonium format, formic acid, and calcium chloride) were supplied by Merck KGaA International (Sigma origin). The EN-QuEChERS (Quick, Easy, Cheap, Efective, Rugged, and Safe) kit for extraction and clean-up of soil samples were procured form Agilent Technologies (Santa Clara, CA 95051, USA). The physicochemical properties of two studied pesticides is presented in Table 1.
Soil and organic amendments
Two typical types of soil including sandy loam, and loam were used in this soil column experiment. The initial soil samples were collected from the agricultural field of Jeollabuk-do, Republic of Korea. The sampling depth is 0–15 cm surface soil including rhizosphere soils. The randomized initial soil samples are mixed and dried under shade avoiding direct sunlight. The compound soil samples were passed through 2.00 mm sieve and stored at room temperature for further use. Amongst the soil's physical characteristics, the soil texture was defined using the “Soil Texture Calculator” derived from the USDA soil textural triangle (USDA, 2022). The soil types were classified as ‘sandy loam’ and ‘loam’ for this study. The soil pH was determined using a digital pH meter following the soil: solution (1:5) while 10 mM CaCl2 was used as the solution instead of distilled water. The physicochemical feature of studied soils are available in Table S1.
As organic amendment (OA), plant litter derived organic fertilizer (OF) was used in this experiment. It is noted that the organic fertilizer is composted product mostly used by the farmers. The reason for using this organic fertilizer is to resemble the real field practice. In addition, a detailed illustration of soil sample preparation is available in the supplementary file (Fig. S1). Furthermore, the soil organic carbon (%OC) was measured following the wet oxidation-modified spectrometric method. The addition of organic fertilizer is favorable for enhanced organic carbon in the soils. The details of the soil treatments and standard methods for soil pH and organic carbon determination are available in the supplementary file (Section S1).
Soil column preparation
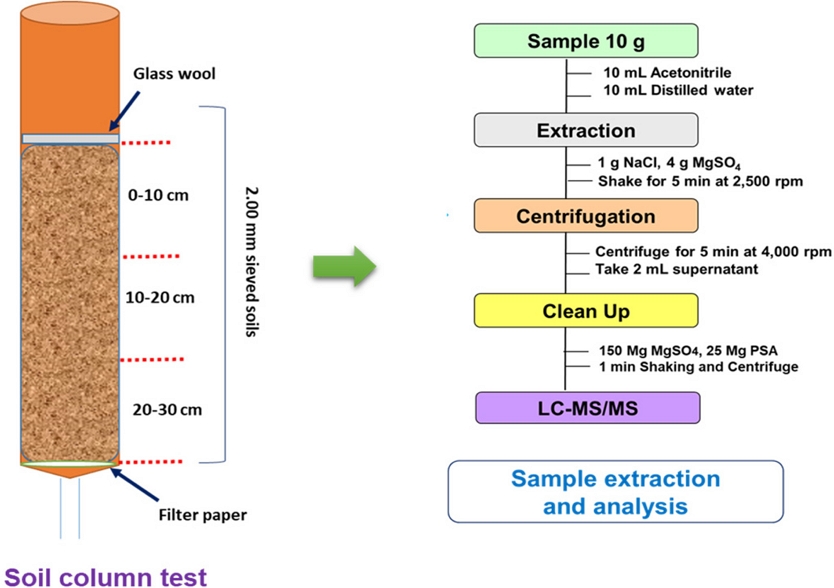
The packing of soil column was performed following the OECD guidelines 312 for “Leaching soil column” (OECD, 2004). At first, the PVC-made laboratory column (height 50 cm, and diameter 5 cm) were filled with approximately 850 g of soil until 30 cm height. It is noted that a qualitative filter paper (Whatman no 1) was placed at the bottom of the each column hindering the soil washout while allowing the water flow. After that, the 1 cm from the bottom of column was filled with quartz sand. During soil column packing the 2.00 mm sieved soils were packed by using spoon, manual vibrator, gentle tapping for avoiding big stubbles, and crack or bubbles of the soil column. After filling the soil column, a glass wool was placed on the top to cover the surface layer of soil column. This glass wool was adventitious for even distribution of artificial rain (0.01 molL-1 CaCl2 solution) throughout of experimental period. Unless specified otherwise, two column sets were prepared for each type of soils. The first column was a control column without any organic amendment (Column A), and the second column B (amended with 2% organic fertilizer), respectively (Fig. 1). This similar method is followed for all studied soil types including sandy loam, and loam soils, respectively.
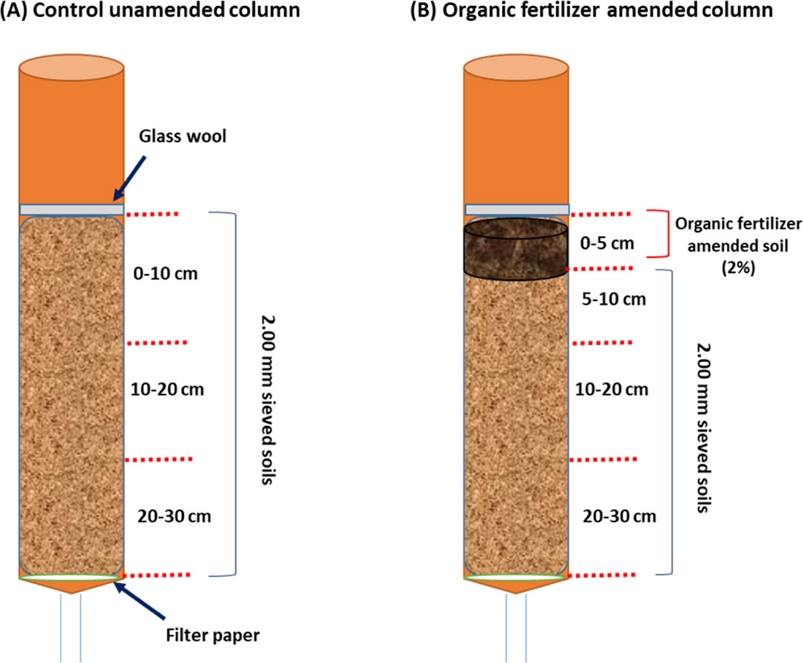
Schematic showing the preparation of column sets for the pesticide leaching tests following the OECD guidelines; A) control unamended column, B) 2% organic fertilizer amended column. This set of column is prepared for a total of two soil types including sandy loam, and loam.
After filling, the soil columns were placed inside the water bucket for saturation of the column soil through capillary water. As soon as the ascending water level was visible at the surface layer, the over saturated column were then removed from the bucket and settled on a plastic holder allowing the excess water to drain naturally. Thus, the saturated column will carry the field capacity water after 24 hours natural drainage.
Pesticide spiking and leaching test
The spiking of 50 μL stock solution (1000 mgL-1) of two studied pesticides (e.g., phorate and terbufos) for each soil column was performed. After spiking, a calculated initial concentration of each pesticide was confirmed 60 μg/g of soil. A glass wool cover is placed at the top of column for ensuring proper and homogeneous distribution of added artificial rain (0.01 molL-1 CaCl2).
Soil column leaching experiment was set in duplicate, a total of eight columns of two soil texture were set for continuous water flow system (addition of 50 mL artificial rain per day) and the columns were leached with 600 mL of solution at a corresponding rate to allow approximately 1 cm water head over the surface throughout the experiment with natural draining facility following the previous investigation (Singh et al., 2003). For sampling, the continuous water flow columns leachate was collected in about 50 mL fractions. At the end of leaching test, each leachate was analyzed through LC-MS/MS for concentration of phorate and terbufos including their five oxidative metabolites.
Sorption and Half-life study of pesticides
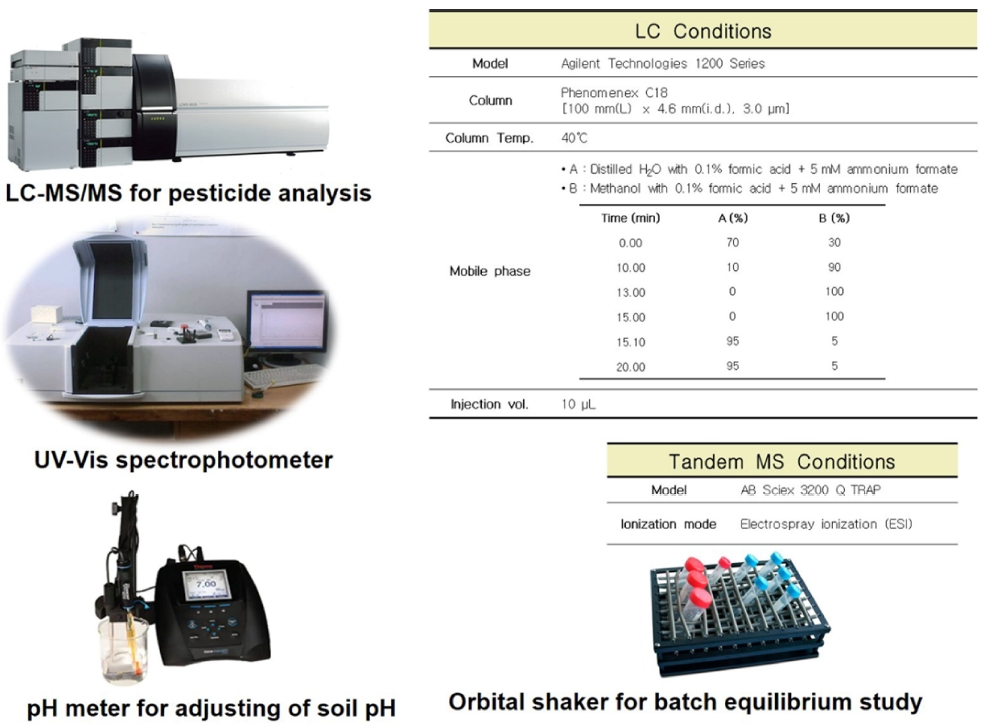
The sorption study of two pesticides (i.e., phorate and terbufos) in two representative soil textures including sandy loam (SL) and loam (L) soils (out of three soil texture) was performed to explore the correlation between sorption potential and leaching of pesticides. A classical batch experiment following the OECD guidelines for adsorption-desorption using a batch equilibrium method (test no. 106) (OECD, 2000). Briefly, the reaction mixture including 2.0 g soil and a specific concentration of pesticides (5.0 mg/kg) in 10 mM CaCl2 (soil: solution = 1:5) was set through batch equilibrium settings following the OECD instructions. The sampling was done at different time intervals (i.e., 12 hours, 18 hours, and 24 hours) to assess the adsorption kinetics over time (Chen et al., 2004). Adsorption parameters were more closely evaluated by the best-fitted sorption isotherms. Among the popular adsorption isotherm, Langmuir and Freundlich's isotherms were widely used for the evaluation of sorption potentiality. These study findings were well-fitted with Freundlich isotherm parameters for explaining the sorption parameters. The Freundlich constant (Kf) denotes the degree of adsorption or, desorption, and 1/n values denote the non-linearity of sorption isotherm (1/n values were within the range of 0.7 to 1.0) for pinpointing the types of sorption.
A fast pseudo-equilibrium was achieved within 18 hours and the strong adsorption potential of studied pesticides was evident by the soil surfaces. Further a comparison of leaching and adsorption of pesticides was assessed. The effect of soil organic carbon (OC) was explained through calculation of KOC value for each soil treatments. Similarly, the degradation and persistence of two pesticides were evaluated through lab incubation assay following the OECD guidelines. Briefly, the soil treatments including control and amended soils (100 g) for each treatment was measured. The specified concentration (5.0 mg/kg) of pesticides (e.g., phorate and terbufos) was spiked to the treated flasks. The reaction mixture was kept for overnight for evaporation of solvent. After that, the treated samples were kept into the lab incubator. The sample was extracted at seven days intervals for determination of degradation half-life (DT50). The calculated degradation DT50 was performed by using first order kinetics equation (DT50 = 0.693/k), where k was the first order kinetic constant.
Extraction of soil column
After leaching of soil columns, the collected leachate at stipulated time was filtered through analyzed without any further extraction. However, the soil columns after leaching was transferred to the freezing (-20oC) for 12 hours. Further, the freezing columns were sectioned into three distinct segments having 10 cm of soil depths for each segment and air-dried for 24 hours. A QuEChERS extraction (EN kit) of the column soils (separated at various depth) was performed. Briefly, 10 g soil sample was placed into a conical tube and 10 mL distilled water and 10 mL acetonitrile was added and shaking was continued for 10 minutes. The extracted pesticide is separated from the water by adding QuEChERS extraction pouch and centrifuged samples were cleaning up using dSPE kit. It is noted that no strong cleanup materials (e.g., C18, GCB, or PSA) is added for avoiding any potential loss of transformed metabolites during this leaching experiment. A detailed diagram of extraction is available in supplementary file.
Calculation of sorption and leaching parameter
Among the adsorption parameters, the distribution coefficient (Kd) is the classical one to denote the distribution of applied pesticide within the soil solid and solution. The calculation of Kd is derived from the following equation no (i):
| (i) |
- Where,
- Kd = distribution coefficient (for linear adsorption; 1/n = 1)
- Cs = Adsorbed amount of pesticide onto soil solid (mg/kg)
- Ce = Equilibrium amount of pesticide in soil solution (mg/l)
However, the adsorbed amount of pesticide onto the soil solid (Cs) can be calculated by the following equation no (iii):
| (ii) |
- Where,
- x = adsorbed amount of pesticide (mg) onto the soil surface
- m = mass of soil sample (kg)
The Freundlich coefficient (Kf) is calculated from the following Freundlich equation (iii):
| (iii) |
- Where,
- Cs = amount of adsorbed pesticide in soil (mg/kg) (derived from equation number ii)
- Ce = equilibrium concentration of pesticide in solution (mg/L)
- Kf = Freundlich sorption constant ((mg/kg)/(mg/L)1/n)
- 1/n = adsorption coefficient (a measure of non-linearity of isotherm)
The Freundlich sorption constant (Kf) is normalized with the amount of organic carbon present in soils for determination of KOC value. The calculation of Koc is derived from the following equation (iv):
| (iv) |
- Where,
- KOC= Organic carbon (OC) normalized partition coefficient
- Kf = Freundlich sorption constant
- foc = amount of organic carbon in soil
In general, the KOC value can be calculated using the Kd value from equation (i) when the isotherm is linear and the 1/n value is 1. However, while the sorption isotherm is for hydrophobic pesticide (i.e., 1/n ≤ 1, indicating the non-linearity of isotherm), the Freundlich constant (Kf) is used instead of Kd for calculating the KOC value.
The leaching potential is calculated by Groundwater Ubiquity Score (GUS) equation (v), and Leachability Index (LIX) equation (vi)
| (v) |
- (GUS > 2.8: Mobile; 1.8 > GUS < 2.8: Transition; GUS < 1.8: Immobile)
| (vi) |
- (LIX = 1: Very mobile; 0.1 > LIX < 1: Mobile; 0 > LIX < 0.1: Transition; LIX = 0: Immobile)
- Where,
- k = first order rate constant
- t½ = half-life (days) of pesticide
- KOC = organic matter normalized coefficient
Instrumental analysis
The simultaneous analysis of parent pesticide (e.g., phorate and terbufos) including their five metabolites was accomplished by liquid chromatography (LC) (Agilent Technologies 1200 series) coupled with tandem mass spectrometry (MS/MS) (AB Sciex 3200 Q TRAP). A Phenomenex C18 column (100 mm [L] × 4.6 mm [internal dia.], 3 μm) was used as a stationary phase analytical column. The gradient mode mobile phase comprising distilled water (DW) and methanol (CH3OH) with 0.1% formic acid and 5 mM ammonium formate is used for this simultaneous pesticide analysis. Additionally, the MS/MS ionization is electrospray ionization (ESI) was conducted in positive mode. The detailed instrumental parameter was presented in the supplementary file (Fig. S3). Additionally, the MRM parameter for the simultaneous analysis of terbufos, phorate and their metabolites by LC-MS/MS is available in the supplementary Table S2. In addition, the analytical parameters including precision, recovery, LOD, and LOQ are available in supplementary file (Table S3).
Statistical analysis
The calculation of adsorption and leaching parameters were performed using specified equation in MS excel (MS office 365). In addition, the graphs and bar diagram showing error bar denoting the standard error (SE) of mean (n = 3) value using MS excel data analysis mode. The rectification of figures were performed by Sigmaplot version 13.0.
Results and discussion
Distribution of pesticides and their metabolites in soil column under varied soil texture
The distribution of phorate and terbufos pesticides along with their metabolic transformation in the different depth of soil columns is illustrated in the following figures (Fig. 2). According to Fig. 2a-2b, in sandy loam soil, the distribution of two pesticides along with metabolic transformation was marked within the 0–10 cm soil depth, while no pesticide/metabolite was detected in the deeper soil zone (e.g., 10-30 cm of column depth). In particular, Fig. 2a showing no detection of parent pesticide and less metabolism with predominant oxidative metabolite including sulfoxide and sulfone in the unamended control soil column without any organic amendment. It is noted that the detection of phorate sulfone in small amount at 10-20 cm column depth indicating the moderate mobility of phorate sulfone into deeper soil layer due to macro pore effect of sandy soils. In contrast, Fig. 2b showing the existence of parent pesticides (e.g., phorate and terbufos) with enhanced metabolism for a highest concentration of phorate sulfone followed by phorate sulfoxide. In contrast, the concentration of terbufos sulfoxide was dominated over terbufos sulfone.
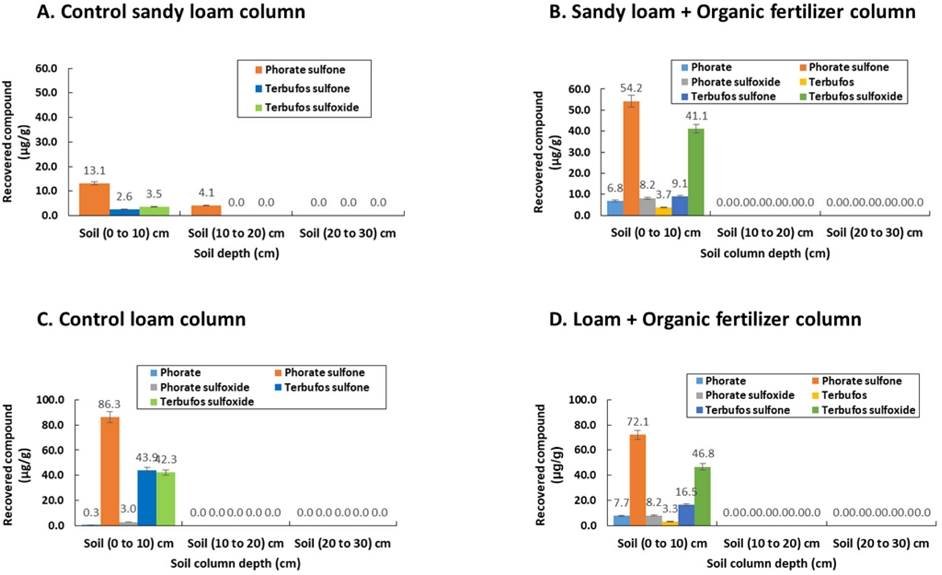
Distribution of phorate and terbufos in different depth of soil column including their major metabolic transformation; A. Control sandy loam column; B. Sandy loam + Organic fertilizer column; C. Control loam column; and D. Loam + Organic fertilizer column.
Likewise, Fig. 2c-2d depicted the distribution of studied pesticides and metabolic transformation in the loam soil. Although, there was no significant difference of pesticide distribution and metabolic transformation between the sandy loam and loam soils except an enhanced metabolism and release of parent pesticides from potential bound residue was documented. Fig. 2c displayed phorate sulfone as the predominant metabolite, whereas terbufos underwent a metabolism to sulfoxide and sulfone simultaneously. Furthermore organic amendment by organic fertilizer could be the triggering factors for enhanced metabolism and release of parent pesticides from the soil-organic amendment matrix (Fig. 2d). However, the specific mechanism is still unknown and can be an interesting area for future research.
On the other hand, the total distribution of phorate and its metabolites and terbufos and its metabolites was presented in Fig. 3. According to Fig. 3, the vadose zone comprising 0-10 cm top soil layer was marked as active zone for distribution and major metabolism of the studied pesticides. In general, the solubility of pesticide in water was related with sorption potential in the soil surfaces. The more strongly adsorbed pesticide were rarely to be leached into deeper soil layer and groundwater. Thus, hydrophobicity of pesticides, solubility in the water, and soil-pesticide interaction all together affecting the pesticide mobility and distribution in the various depth of soil column. Finally, the soil texture and compatibility of soil-organic amendment were acting as the key triggering factors for controlling distribution and mobility of hydrophobic pesticides into the soil layers.
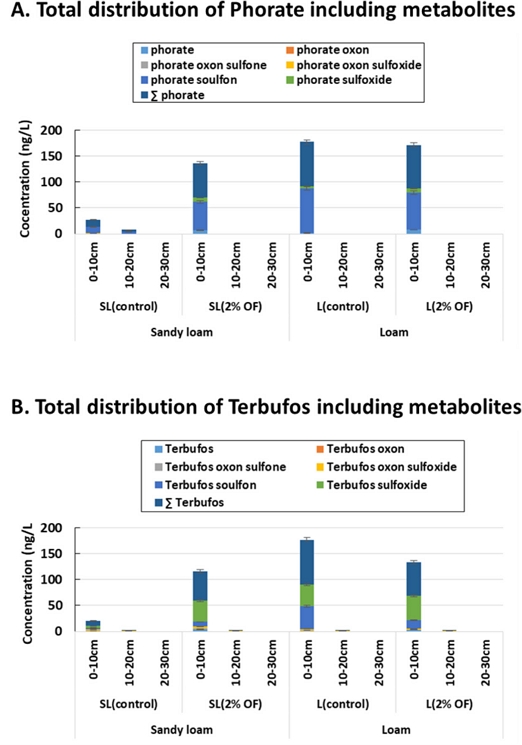
Total distribution of studied pesticides including phorate (A) and terbufos (B) in various depth of soils; the vadose zone (0-10 cm) showing the active zone for pesticide distribution and metabolic transformation.
According to above figures (Fig. 2 and Fig. 3), the distribution of studied pesticides (e.g., phorate and terbufos) is confined within the vadose zone (0-10 cm soil column layer). However, the oxidative metabolism and oxidative transformation products (i.e., sulfoxide and sulfone) were dominating over the other minor metabolic transformation products. Previous studies deciphering the triggering factors such as pesticide solubility, interaction of soil with pesticides, and adsorption potential to control the leaching behavior and mobility of pesticides within soil column (Katagi, 2013). In addition, the adsorption of hydrophobic pesticides in the top soil layer is negatively correlated with leaching and mobility of the pesticides and the level of organic matter in soil have significant impact during adsorption and mobility of non-ionic, and hydrophobic pesticides (Gaonkar et al., 2019). Therefore, further meticulous field and laboratory experiments should be designed to explore the detailed insight of pesticide persistence, metabolism, and mobility within the soil profile.
Role of sorption and KOC during mobility of pesticides
Amongst the soil processes, soil sorption of pesticide is principally controlling the mobility and leaching behavior of pesticide. The solubility of pesticide is the key indicator for sorption by the soil matrix. In this study, two studied pesticide having the very poor solubility tends to be adsorbed by the soil matrix. As a result, the leaching and mobility of pesticide is limiting by this sorption effect. According to the physicochemical properties of the two pesticides (terbufos, and phorate), the hydrophobicity of terbufos (4.48) was found to be higher than phorate (3.56). Therefore, the strong adsorption of phorate and terbufos was documented by the soil matrix regardless of soil texture and organic amendments. The distribution coefficient (Kd) and sorption coefficient normalized with the organic matter content of soil (KOC) are two vital parameters for elucidating the sorption behavior of hydrophobic pesticides onto soils. The Kd and KOC values of terbufos and phorate derived from this study are summarized in Table 2. It is noted that Kd is the ratio of the initial and equilibrium concentration of the studied pesticide, which is derived from the linear isotherm (where the 1/n value is 1.0); whereas the KOC value is derived from the Kf (Freundlich isotherm constant) (where 1/n ≤ 1.0). In this study, the Freundlich isotherm constant (Kf) is used for calculating the KOC value, because the sorption isotherm for terbufos and phorate is nonlinear (L-type isotherm). The L-type nonlinear sorption isotherm indicative of strong adsorption of phorate and terbufos by the studied soils.

Distribution coefficient (Kd) and Koc values of the studied soil treatments for two non-ionic pesticides in two representative soil textures including sandy loam (SL) and loam (L) soil. Plant leaf derived organic fertilizer (OF) was used as a cheap organic amendment
It was earlier well explained that the soil pH has no significant impact during the adsorption-desorption behavior of non-ionic pesticides (Chen et al., 2004; Rani and Juwarkar 2010), whereas the dissipation, and sorption of ionic pesticides were mediated by the soil pH, organic matter contents, clay types and temperature of soils (Chen et al., 2018; García-Delgado et al., 2020). In general, soil organic carbon, clay types, and soil pH are the triggering factors during the sorption and mobility assessment of pesticides (Benoit et al., 2008; Chen et al., 2018). But the effect of soil pH and clay types may not be applicable to non-ionic pesticides sorption behavior without a few exceptions (Gondar et al., 2013; Carpio et al., 2021). Thus, the effect of organic carbon on the sorption of hydrophobic pesticides was more closely explained by the KOC (organic matter normalized Kf) as a key universal sorption parameter (Dos Reis et al., 2014). In this study, the KOC value (derived from the equation iv), has shown a positive correlation with the degree of sorption for non-ionic pesticides under varied soil treatment conditions. The addition of organic fertilizer (OF) has a positive effect on increased Koc value and subsequent sorption onto soils (Table 2). It is noted that the cheap source organic fertilizer is used in this study to resemble the real field conditions as farmer’s practice. Previous studies have documented the positive correlation between the addition of organic amendment and enhanced sorption of pesticides onto soils (Deng et al., 2017; Mendes et al., 2019; Carpio et al., 2021), resulting in decreased leaching loss and least mobility of pesticides through the soil profile. As a whole, the strong sorption of pesticide have a negative correlation for pesticide mobility and leaching.
Persistence, degradation, and metabolic transformation
According to persistence data, DT50 values of phorate and terbufos varied significantly by various factors including soil moisture, soil organic matter and sterilization (impact of soil microbes) (Table 3) (data derived and modified from another separate investigation by our research group which is now under review by another journal). The moisture and soil organic matter were found to be the most influential factors for controlling the degradation and persistence of non-ionic and hydrophobic pesticides (i.e., phorate and terbufos). The pesticide properties database (PPDB) having the incomplete information for the half-life values for phorate and terbufos with a wide range of variation due to lack of specific research data concerning diversified growing conditions and pesticide formulations. However, this study findings will decipher the key factors for wide variation of persistence and degradation patterns of phorate and terbufos using two typical soils including sandy loam, and loam. According to Table 3, soil moisture was considered the initial triggering factors for enhanced degradation of phorate and terbufos. In addition, organic fertilizer (OF) was amended in soils for improving soil quality, and organic carbon status. The OF treated soil showing the enhanced degradation of phorate and terbufos, resulting in reduced half-life (DT50) as compared to control treatment. In contrast, hydrogen peroxide treatment was performed for the partial removal of organic matter, which showing the hindrance of pesticide degradation due to partial removal of organic matter from soils. Thus, hydrogen peroxide treated soils exhibited the more persistence of phorate and terbufos in the soils. Thus, the necessary revision of PPDB database for persistence and degradation of phorate and terbufos can be done using the outcomes of this study with specific factors that controlling the degradation half-life of phorate and terbufos.

Persistence of phorate and terbufos based on degradation DT50 values in Sandy loam and Loam soils under the influence of vital soil factors including moisture, organic matter and soil microbes. The degradation kinetics followed the first order kinetic equation (DT50 = 0.693/k); k is the first order rate
On the other hand, the unamended soil column (control) showing the negligible to no detection of parent pesticides (i.e., phorate or terbufos) whereas, the amended soil column by organic amendments (e.g., plant litter derived organic fertilizer) revealed the parent pesticide’s existence with enhanced metabolism (Fig. 2). On the other hand, immediately after application, phorate and terbufos were rapidly adsorbed by the soil matrix due to the insolubility with water and higher logP values. However, the next steps were including complete mineralization, transformation into metabolites, and chemo-bio degradation by the influence of vital soil- and pesticide factors (Rasool et al., 2022; Shi et al., 2022). Another key factor is formation of bound residue while pesticides were strongly adsorbed by the soil humic materials. This study did not measuring the humic materials of the soil samples. Thus, the uncertainty of bound residue can be explored by further experiment by more robust extraction of bound residue from the treated soils. The detection of parent pesticide (e.g., phorate and terbufos) in the amended soil column should be triggered by the potential release of pesticide from bound residue (Jariyal et al., 2018). Furthermore, the metabolic transformation showing the transformation of phorate and terbufos into sulfoxide and sulfone. In particular, the mostly detected metabolite was sulfone regardless of phorate and terbufos in presence of organic amendments. The enhanced oxidative metabolism of phorate and terbufos could be governed by the activation of soil microbial community and augmentation of organic carbon by organic amendments (Jariyal et al., 2018; Dar et al., 2022).
This study was more closely observed for the active zone of metabolism and degradation of pesticides using soil column sections. The results indicated that, the vadose zone (0–10 cm top layer) was the active zone for pesticide degradation and metabolism, where most of the metabolites and residual parent pesticides (i.e., phorate and terbufos) were detected. In contrast, no detectable pesticides and metabolites was observed in the deeper soil column layers (e.g., 10–20 cm, and 20–30 cm column depth) during this column study. Additionally, sulfone metabolite of both pesticides including phorate and terbufos was detected as the prominent and persistent metabolite in the surface soil layer of soil column only.
Leaching behavior and mobility
The leaching behavior of two studied pesticides (phorate and terbufos) was evaluated under continuous flow of waters. Regardless of the water flow systems, the both pesticides were termed as non-leacher due to high sorption potential with soil matrix and insolubility with soil water. Thus, no parent pesticides or their transformed metabolites were detected in the leachate. The leaching potential was assessed by using GUS (see eq. v) and LIX (see eq. vi) equations for phorate, terbufos and their major metabolites. According to GUS and LIX index, both pesticides and their metabolites were categorized as “immobile”. In particular, based on the leachability index, sandy loam soil showing extremely low leachability, while loam soil showing the low leachability as per GUS leaching classifications. The leaching index and respective category of studied pesticide and their metabolites is presented in Table 4. According to Table 4, the studied pesticides (e.g., phorate and terbufos) showing immobility while performing soil leaching investigation indicated that there is no potential risk of groundwater contamination through parent compound of phorate and terbufos. Besides, during collection of 450 mL to 600 mL leachate, there are some metabolite (e.g., sulfone) found in trace amount but the GUS and LIX index of total pesticide residue showing the “immobile” or extremely low leachability nature. Due to strong sorption potential with soil matrix, no potential risk was predicted for groundwater contamination for phorate and terbufos. However, the slightly mobile and detected metabolite can cause a potential groundwater contamination. Thus, a separate metabolite persistence and mobility study should be explored further to resolve this research uncertainty.

Leaching indicator through GUS (equation v) and LIX (equation vi) index of the pesticide phorate and terbufos in various soils and their respective mobility
A previous study noticed two pesticides out of four studied pesticides as immobile and the metabolic transformation was evident within the soil column during a disturbed column experiment (Aliste et al., 2021). The persistence of the studied pesticide In contrast, during the column study, chlorpyrifos and cypermethrin were found as the immobile and less prone to the leachate (Rani et al., 2004). The majority of the detected pesticides were found in the top 10 cm soil column zone. Thus, the vadose zone including 0-10 cm soil column was considered active zone for hydrophobic pesticides. In general, leaching of pesticides depends on the pesticide solubility, hydrophobicity, and adsorption potential of pesticides with soil surfaces. The pesticide which attached to the soil surfaces more strongly have less chance to leach through groundwater.
Limitations and future recommendations
The packed soil column experiment is not reflecting the real field data for pesticide mobility and leaching potential due to soil textural heterogeneity and climatic factors (Aliste et al., 2021; Xie et al., 2021). Thus, the outcomes of the laboratory column experiment regarding pesticide distribution, persistence, metabolism, and leaching behavior is not directly applied for the real field conditions, rather can be used as the fundamental basis for pesticide behavior under varied soil texture, organic amendments, and water flow patterns. This is the vital research gap of packed soil column experiment. The following recommendations are suggested to explore the pesticide monitoring and mobility in the field soils with respect to packed soil column findings:
- 1. In this investigation, no residual phorate and terbufos was detected in the leachate and that was not causing groundwater contamination according to GUS index. As a result, surface water monitoring is recommended for phorate and terbufos to expedite the potential pesticide run-off instead of groundwater leaching.
- 2. According to the study result, the distribution of phorate and terbufos was limited within the top 10 cm soil column depth. It is indicative of the limited distribution within soil profile and persistence of phorate and terbufos mostly evident at the vadose zone soil (i.e., 0–10 cm topsoil). Thus, further dissipation, monitoring and persistence study of phorate and terbufos including transformed metabolites should be explored in the vadose zone soils.
- 3. In this study, metabolite transformation in the different soil depth is explored but the specific persistence (half-life) was not studied. Thus, meticulous studies is suggested to monitor the persistence and mobility of metabolites of phorate and terbufos in diversified soil, sediment and leachate samples.
- 4. As the solubility of pesticides (e.g., phorate and terbufos) was very low in water, a major portion of applied pesticide was either mineralized, transformed into metabolites, or remain in the soil as bound residue at the vadose zone of soil. This kind of behavioral uncertainty of phorate and terbufos should be investigated by further extraction of bound residue into the soil solution. Briefly, fates of phorate and terbufos can be explored in details including potential metabolism in the surface region of soils.
- 5. This investigation was confined within two types of soils (e.g., sandy loam, and loam) and the representative and cheap organic amendments (plant litter derived organic fertilizer). Further experiment can be extended using diversified soils and organic amendment in real field settings under varied environmental factors.
Conclusion
In summary, the persistence, mobility and distribution of phorate and terbufos was controlled by several soil factors including soil texture, soil moisture, and organic amendments. The persistence of phorate and terbufos was not significantly varied for sandy loam and loam soil texture; whereas the triggering factors such as soil moisture, addition/removal of organic matter, and soil sterilization were found to be the vital ones for controlling persistence of phorate and terbufos. In the packing soil column investigation, the distribution of parent pesticides (e.g., phorate and terbufos) was confined within the top soil layer (soil column depth 0 to 10 centimeter). In addition, the transformation of parent pesticides into major metabolites was also evident at those vadose zone soils. Among the transformed metabolites, sulfoxide and sulfone were predominating. In particular, phorate sulfone was dominating over phorate sulfoxide; whereas terbufos sulfoxide dominating over terbufos sulfone. In contrast, the GUS index deciphering the studied pesticides including phorate and terbufos as ‘immobile’ with low leachability indicating no potential risk of groundwater contamination, but a slightly mobile metabolite can cause groundwater contamination. The total distribution of phorate and terbufos showing the top 10 centimeter soil layer (i.e., vadose zone soil) as active zone for distribution of parent pesticides and transformation into key metabolites. Thus, monitoring of field soils and pesticide surface runoff studies are recommended to explore the detailed fate and distribution of phorate and terbufos under real field conditions.
Acknowledgments
Authors are grateful to National Institute of Agricultural Sciences for supporting the lab and analysis. This study was supported by 2023 the RDA Research Associate Fellowship Program of National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea, grant number PJ015944.
References
-
Aliste M, Pérez-Lucas G, Garrido I, Fenoll J, Navarro S, 2021. Mobility of insecticide residues and main intermediates in a clay-loam soil, and impact of leachate components on their photocatalytic degradation. Chemosphere 274.
[https://doi.org/10.1016/j.chemosphere.2021.129965]

-
Barba V, Marín-Benito JM, Sánchez-Martín MJ, Rodríguez-Cruz MS, 2020. Transport of 14C-prosulfocarb through soil columns under different amendment, herbicide incubation and irrigation regimes. Sci. Total Environ. 701:134542.
[https://doi.org/10.1016/j.scitotenv.2019.134542]

-
Bindumol GP, Harilal CC, 2017. Mobility and dissipation of chlorpyriphos and quinalphos in sandy clay loam in an agroecosystem-a laboratory-based soil column study. Environ. Monit. Assess. 189(5).
[https://doi.org/10.1007/s10661-017-6142-9]

-
Cara IG, Țopa D, Puiu I, Jităreanu G, 2022. Biochar a promising strategy for pesticide-contaminated soils. Agriculture 12(10):1579.
[https://doi.org/10.3390/agriculture12101579]

-
Carpio MJ, Sánchez-Martín MJ, Rodríguez-Cruz MS, Marín-Benito JM, 2021. Effect of organic residues on pesticide behavior in soils: a review of laboratory research. Environments 8(4):32.
[https://doi.org/10.3390/environments8040032]

-
Cueff S, Alletto L, Bourdat-Deschamps M, Benoit P, Pot V, 2020. Water and pesticide transfers in undisturbed soil columns sampled from a Stagnic Luvisol and a Vermic Umbrisol both cultivated under conventional and conservation agriculture. Geoderma 377:114590.
[https://doi.org/10.1016/j.geoderma.2020.114590]

-
Dar MA, Baba ZA, Kaushik G, 2022. A review on phorate persistence, toxicity and remediation by bacterial communities. Pedosphere 32:171-183.
[https://doi.org/10.1016/S1002-0160(21)60043-7]

-
Fouad MR, 2023. Effect of peat, compost, and charcoal on transport of fipronil in clay loam soil and sandy clay loam soil. Curr. Chem. Lett. 12(2):281-288.
[https://doi.org/10.5267/j.ccl.2022.12.011]

-
Gaonkar OD, Nambi IM, Govindarajan SK, 2019. Soil organic amendments: impacts on sorption of organophosphate pesticides on an alluvial soil. J. Soils Sediments 19:566-578.
[https://doi.org/10.1007/s11368-018-2080-6]

-
Hall KE, Ray C, Ki SJ, Spokas KA, Koskinen WC, 2015. Pesticide sorption and leaching potential on three Hawaiian soils. J. Environ. Manage. 159:227-234.
[https://doi.org/10.1016/j.jenvman.2015.04.046]

-
Holten R, Larsbo M, Jarvis N, Stenrød M, Almvik M, et al., 2019. Leaching of five pesticides of contrasting mobility through frozen and unfrozen soil. Vadose Zo. J. 18(1):1-10.
[https://doi.org/10.2136/vzj2018.11.0201]

-
Jariyal M, Jindal V, Mandal K, Gupta VK, Singh B, 2018. Bioremediation of organophosphorus pesticide phorate in soil by microbial consortia. Ecotoxicol. Environ. Saf. 159:310-316.
[https://doi.org/10.1016/j.ecoenv.2018.04.063]

-
Katagi T, 2013. Soil column leaching of pesticides, Reviews of Environmental Contamination and Toxicology. 221:1-105.
[https://doi.org/10.1007/978-1-4614-4448-0_1]

-
Khorram MS, Wang Y, Jin X, Fang H, Yu Y, 2015. Reduced mobility of fomesafen through enhanced adsorption in biochar-amended soil. Environ. Toxicol. Chem. 34(6):1258-1266.
[https://doi.org/10.1002/etc.2946]

-
Kumari U, Singh SB, Singh N, 2020. Sorption and leaching of flucetosulfuron in soil. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes 55(6):550-557.
[https://doi.org/10.1080/03601234.2020.1733363]

-
Magga Z, Tzovolou DN, Theodoropoulou MA, Dalkarani T, Pikios K, et al., 2008. Soil column experiments used as a means to assess transport, sorption, and biodegradation of pesticides in groundwater. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes 43(8):732-741.
[https://doi.org/10.1080/03601230802388868]

-
Manna S, Singh N, 2019. Biochars mediated degradation, leaching and bioavailability of pyrazosulfuron-ethyl in a sandy loam soil. Geoderma 334:63-71.
[https://doi.org/10.1016/j.geoderma.2018.07.032]

-
Marín-Benito JM, Brown CD, Herrero-Hernández E, Arienzo M, Sánchez-Martín MJ, et al., 2013. Use of raw or incubated organic wastes as amendments in reducing pesticide leaching through soil columns. Sci. Total Environ. 463-464:589-599.
[https://doi.org/10.1016/j.scitotenv.2013.06.051]

-
Marín-Benito JM, Herrero-Hernández E, Ordax JM, Sánchez-Martín MJ, Rodríguez-Cruz MS, 2021. The role of two organic amendments to modify the environmental fate of S-metolachlor in agricultural soils. Environ. Res. 195:110871.
[https://doi.org/10.1016/j.envres.2021.110871]

-
Mendes KF, de Sousa RN, Takeshita V, Alonso FG, Régo APJ, et al., 2019. Cow bone char as a sorbent to increase sorption and decrease mobility of hexazinone, metribuzin, and quinclorac in soil. Geoderma 343:40-49.
[https://doi.org/10.1016/j.geoderma.2019.02.009]

-
Muendo BM, Shikuku VO, Getenga ZM, Lalah JO, Wandiga SO, et al., 2021. Adsorption-desorption and leaching behavior of diuron on selected Kenyan agricultural soils. Heliyon 7:e06073.
[https://doi.org/10.1016/j.heliyon.2021.e06073]

- OECD, 2000 - Organisation for Economic Co-operation and Development, 2000. OECD Guidelines for the Testing of Chemicals. Test Number 106, Adsorption – Desorption Using a Batch Equilibrium Method, OECD: Paris. (44 pp).
-
OECD, 2002- Organisation for Economic Co-operation and Development, 2002. Test No. 307: Aerobic and Anaerobic Transformation in Soil.
[https://doi.org/10.1787/9789264070509-en]

-
Pérez-Lucas G, El Aatik A, Vela N, Fenoll J, Navarro S, 2021. Exogenous organic matter as strategy to reduce pesticide leaching through the soil. Arch. Agron. Soil Sci. 67(7):934-945.
[https://doi.org/10.1080/03650340.2020.1768531]

-
Pérez-Lucas G, Gambín M, Navarro S, 2020. Leaching behaviour appraisal of eight persistent herbicides on a loam soil amended with different composted organic wastes using screening indices. J. Environ. Manage. 273:111179.
[https://doi.org/10.1016/j.jenvman.2020.111179]

-
Rani M, Saini S, Kumari B, 2014. Leaching behaviour of chlorpyriphos and cypermethrin in sandy loam soil. Environ. Monit. Assess. 186:175-182.
[https://doi.org/10.1007/s10661-013-3364-3]

-
Rani R, Juwarkar A, 2010. Adsorption of phorate, an organophosphorus pesticide, on vertisol. Arch. Environ. Contam. Toxicol. 58:927-934.
[https://doi.org/10.1007/s00244-009-9424-6]

-
Rasool S, Rasool T, Gani KM, 2022. A review of interactions of pesticides within various interfaces of intrinsic and organic residue amended soil environment. Chem. Eng. J. Adv. 11:100301.
[https://doi.org/10.1016/j.ceja.2022.100301]

-
Sarkar S, Mukherjee I, 2021. Effect of organic amendment on mobility behavior of flupyradifurone in two different Indian soils. Bull. Environ. Contam. Toxicol. 107:160-166.
[https://doi.org/10.1007/s00128-021-03209-4]

-
Sarker A, Islam T, Rahman S, Nandi R, Kim JE, 2021a. Uncertainty of pesticides in foodstuffs, associated environmental and health risks to humans-a critical case of Bangladesh with respect to global food policy. Environ. Sci. Pollut. Res. 28:54448-54465.
[https://doi.org/10.1007/s11356-021-16042-3]

-
Sarker A, Lee SH, Kwak SY, Nam AJ, Kim HJ, et al., 2020. Residue monitoring and risk assessment of cyazofamid and its metabolite in Korean cabbage under greenhouse conditions. Bull. Environ. Contam. Toxicol. 105:595-601.
[https://doi.org/10.1007/s00128-020-02972-0]

-
Sarker A, Nandi R, Kim JE, Islam T, 2021b. Remediation of chemical pesticides from contaminated sites through potential microorganisms and their functional enzymes: Prospects and challenges. Environ. Technol. Innov. 23:101777.
[https://doi.org/10.1016/j.eti.2021.101777]

-
Shi X, Zhang W, Bian C, Li B, 2022. Adsorption–desorption and migration behaviors of oxaziclomefone in different agricultural soils in China. Bull. Environ. Contam. Toxicol. 108:791-800.
[https://doi.org/10.1007/s00128-022-03457-y]

-
Singh, N., Singh, B., Dureja, P., Sethunathan, N., 2003. Persistence of phorate in soils: role of moisture, temperature, preexposure, and microorganisms. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes 38(6):723-735.
[https://doi.org/10.1081/PFC-120025556]

-
Song NH, Chen L, Yang H, 2008. Effect of dissolved organic matter on mobility and activation of chlorotoluron in soil and wheat. Geoderma 146(1-2):344-352.
[https://doi.org/10.1016/j.geoderma.2008.05.031]

- USDA, 2022. Soil Texture Calculator. https://www.nrcs.usda.gov/resources/education-and-teaching-materials/soil-texture-calculator, . (Accessed May 30, 2023)
-
Varjani S, Kumar G, Rene ER, 2019. Developments in biochar application for pesticide remediation: Current knowledge and future research directions. J. Environ. Manage. 232:505-513.
[https://doi.org/10.1016/j.jenvman.2018.11.043]

-
Wang XQ, Liu J, Zhang N, Yang H, 2020. Adsorption, mobility, biotic and abiotic metabolism and degradation of pesticide exianliumi in three types of farmland. Chemosphere 254:126741.
[https://doi.org/10.1016/j.chemosphere.2020.126741]

-
Xie G, Li B, Tang L, Rao L, Dong Z, 2021. Adsorption-desorption and leaching behaviors of broflanilide in four texturally different agricultural soils from China. J. Soils Sediments 21:724-735.
[https://doi.org/10.1007/s11368-020-02831-9]

Appendix
Influence of soil types and organic amendment during persistence, mobility, and distribution of phorate and terbufos in soils
Supplementary File
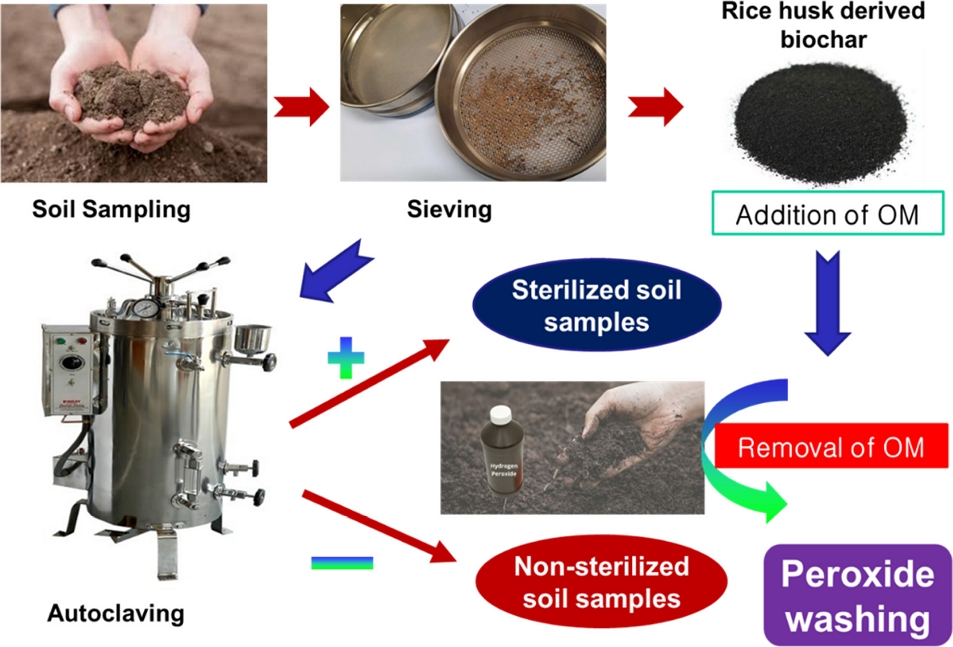
The flowchart showing the soil sample preparation including sieving and autoclaving during adsorption studies using biochar derived from rice husk as an organic amendment.
The compound soil samples were sterilized by autoclaving for two hours after sieving through a 2.00 mm sieve to prevent any microbial degradation of applied pesticides. Autoclaving could be a useful method of sterilization to prevent potential microbial degradation of pesticides. Rice husk biochar (BC) is used in this study as an organic amendment. The slow pyrolysis with high temperature (≥550oC) method-derived biochar (ground particle size ≤ 0.15 mm) is used in this study without any further modification or purifications. Additionally, the organic fertilizer derived from Amongst the soil's physical characteristics, the soil texture was defined using the “Soil Texture Calculator” derived from the USDA soil textural triangle (USDA, 2022). The soil types were classified as ‘Sandy loam’ and ‘Loam’ and ‘Clay loam’ for this study. The soil pH was determined using a digital pH meter following the soil: solution (1:5) while 10 mM CaCl2 was used as the solution instead of distilled water. The chemical characterization of soil treatments is presented in Table S1.
Furthermore, the soil organic carbon (%OC) was measured following the wet oxidation-modified spectrometric method. The addition of biochar is favorable for enhanced organic carbon in the soils. The details of the soil treatments and standard methods for soil pH and organic carbon determination are available in the supplementary file (Section S1)
A simplified flow diagram showing the lab incubation for degradation (A), and adsorption-desorption study (B), for two non-ionic pesticides, following the OECD guidelines by batch equilibrium method.
Instrumental parameter and technical feature of LC-MS/MS during simultaneous analysis of Terbufos and Phorate in investigated soil samples.
Section S1: UV Spectrophotometer Method for determination of Total Organic Carbon in Soils
Apparatus:
1. UV spectrophotometer
2. Volumetric flask
Reagents:
1. 1N potassium dichromate: Dissolve 49.04 AR grade K2Cr2O7 (dry) in distilled water and make up the volume to one liter.
2. 97% conc. Sulphuric acid
Procedure:
Preparation of Standard Curve:
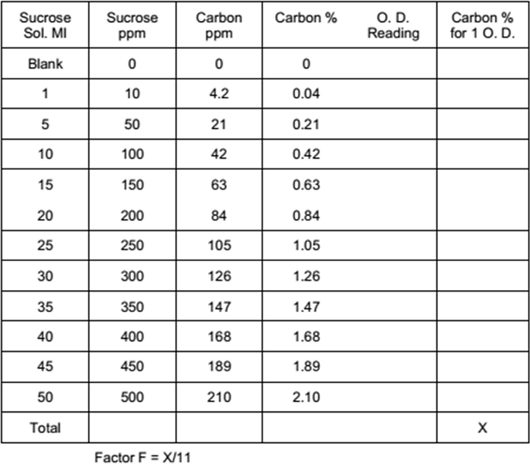
Take 1 gm sucrose and add to it 1000 ml distilled water. From this solution take 0, 1, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50 ml in 100 ml flask and add 10 ml potassium dichromate and 20 ml sulphuric acid. Shake well and allow the mixture to cool on asbestos sheet, Make the volume of each solution to 100 ml with distilled water and observe optical densities at 660 nm wavelength. Prepare standard curve and calculate factor F.
Observation table
Determination of Organic Carbon
Sieve the soil sample with 1 mm sieve and take 1 gm of sieved soil sample in 100 ml flask. Add to it 10 ml potassium dichromate and 20 ml sulphuric acid, shake well and allow it to cool on asbestos sheet. Make the volume to 100 ml with distilled water and keep it overnight. Measure optical density at 660 nm wavelength on spectrophotometer.
Organic Carbon % = Optical density × Factor F
Aniruddha Sarker, Residual Chemical Assessment Division, Department of Agro-Food Safety and Crop Protection, National Institute of Agricultural Sciences, Rural Development Administration, Postdoctoral Research Associate, Conceptualization, Experimental design, Laboratory experiment, Analysis, Writing of manuscript. ORCID: https://orcid.org/0000-0001-6751-7301
Won-Tae Jeong, Residual Chemical Assessment Division, Department of Agro-Food Safety and Crop Protection, National Institute of Agricultural Sciences, Rural Development Administration, Agriculture Researcher, Conceptualization, Experimental design, Review manuscript, Supervision