Residual Characteristics of Fungicide Fenhexamid in Kiwi Fruit during Cultivation and Post-harvest Storage
Abstract
This study was carried out to investigate residual the characteristics of fenhexamid in kiwi during its cultivation and post-harvest storage. The field experiments were conducted in Jeju Special Self-Governing Province, Jeju City, Doryeon-dong. The target pesticides were sprayed twice before the harvest during 30-21, 21-14, 14-7, and 7-0 days. More than 2 kg kiwi were harvested as samples from the treated plants, and saved in deep freezer at -70oC. Prior to analysis, the kiwi were homogenized with a blender, and frozen at -20oC. The fenhexamid was partitioned and extracted from the kiwi with acetone/dichloromethane solvent pair, followed by purification with SPE – FL (1 g), and analysis using with LC-MS/MS. The method limit of quantification (MLOQ) of fenhexamid was 0.01 mg/kg. The recovery of fenhexamid from the kiwi samples at the levels of MLOQ and 10 MLOQ were 103.7 ± 2.8, and 104.0 ± 0.5, respectively. The maximum residue of fenhexamid was 4.69 mg/kg at 0 DAT. The safety guideline proposed in the present study for the use of fenhexamid is two treatments in every seven days before the kiwi harvest. The initial residual level of fenhexamid was 4.32 mg/kg for kiwi samples aged at room temperature and 4.37 mg/kg for the kiwi samples in the refrigerator. After ripening kiwi at room temperature, 4.03, 3.72, 3.64 mg/kg of fenhexamid were found after 3, 6, and 8 days, respectively, which was reduced by 15.7% compared to the initial residual amount. On the other hand, for the kiwi aged in the refrigerator, 4.01, 4.03, 3.91, 4.04, 2.64 mg/kg of fenhexamid were found after 4, 8, 12, 15 and 21 days, respectively, which was about 39.7% less than the initial residual amount. During the same period (8 days), a faster decrease in residue was observed when the kiwi was matured at room temperature than that ripened in the refrigerator. During the same period (8 days), it was confirmed that the residual amount decreased by about 7.7% when aged at room temperature rather than refrigerated aging.
Keywords:
Fenhexamid, Fungicide, Kiwi, Residual pesticide, Post-harvest storageIntroduction
Kiwi cultivation in Korea has been in full swing since the 1990s, and production has skyrocketed since 1999, with facility cultivation prevailing. In addition, cultivation is spreading from Jeju to Gyeongsangnam-do and Jeollanam-do regions due to climate warming and rising consumer preferences (NIHHS, 2017). Consumers who have been exposed to the media reports that pesticides exceeding criteria have been detected in imported fruits that have been on the market for several years are inevitably worried about residual pesticides. In addition, due to the introduction of the Positive List System (PLS), kiwi’s residual pesticide violation rate is expected to increase. However, as the climate and agricultural environment diversify, it is true that various pesticides are inevitable in countries around the world. If pesticides are not used, production will decrease, and the labor required will increase. Pesticides are widely used in agriculture to protect crops.
As reported by the World Health Organization (WHO), pesticides were being developed and released year after year, and their use is increasing worldwide (Lassalle et al., 2014). According to Abd-Alrahman and Arias-Estevez, it is an essential element of modern agriculture, destroying, repelling, or alleviating pests (Abd-Alrahman, 2014; Arias-Estévez et al., 2018). However, Oramed et al. (2008) reported that pesticides are toxic substances that are toxic to living organisms, are difficult to decompose, and have a lasting bio-accumulative effect. Therefore, with increasing concern about food safety and environmental impact, more research is being conducted into the effects of pesticide residues on crops (Abd-Alrahman, 2014; Rozemeijer and Broers, 2007). It also monitors the levels of pesticide residues in domestic and imported agricultural products to ensure consumer health and to prevent the marketing of foods containing residues that exceed certain tolerance limits set by regulations. The widely used pesticides are regarded as important agricultural material in modern agriculture because of the pesticide residue problem major issue not only in terms of protection for agricultural production but also in international trade in agricultural products.
Used this study fenhexamid was noticed developed by Bayer AG of Germany in 1989 (Turner, 2018). Fenhexamid was introduced as a fungicide to control graymold in addition to Botrytis cinerea and as the causative agent of the disease to Sclerotinia sclerotiorum. Also effective in controlling spores such as Monilinia spp (Angioni et al., 2011; Jin et al., 2018; KCPA, 2015). The fenhexamid inhibits the growth of germinal tube kidney and hyphae, mainly grapes and peach, tomato, ginseng, cucumber, apple, rose, etc. It is known a pesticide used to control light spot disease (Angioni et al., 2011; KCPA, 2015). Also, the biosynthesis of ergosterol inhibits, it is efficient to pathogen with resistance. It is resistant to hydrolysis and lasts and is a stable compound with good properties (Esteve-Turrillas et al., 2011). The structure and physicochemical properties are listed in Table 1. This study is conducted to evaluate pesticide residues in kiwi fruit treated with fenhexamid, to use for pesticide registration, and to set safe use guidelines by identifying the tendency of pesticide residue reduction among kiwi with different number of spray and harvest days. Also, through this study, it is to confirm the residual characteristics of fenhexamid according to the storage temperature of kiwifruit, and to contribute to the consumption of safer agricultural products.
Materials and Methods
Chemicals, reagents, and equipment
Fenhexamid (purity 99.3%) was purchased from Sigma Aldrich® Korea. All that acetone, acetonitrile, dichloromethane, and ethyl acetate were used HPLC grade of Brudick & Jackson (Burdick & Jackson, Muskegon, MI, USA). The distilled water used was purified by Zeneer Power II (Human Co., Korea). Acetic acid (EP grade), formic acid (EP grade and purity 95%), sodium hydroxide (EP grade), sodium chloride (EP grade), and anhydrous sodium sulfate (EP grade) were purchased from Samchun chemical Co. (Korea). The SPE (1 g) silica, florisil cartridge was manufactured by Phenomenex (CA, USA). The mixer (NFM-8860) was manufactured by NUC (Korea), the filter paper No. 2 was manufactured by Advantec (Tokyo, Japan), and the rotary evaporator (N110-S) was manufactured by EYELA (Tokyo, Japan). Nitrogen evaporator was Hurricane-Lite from Chongmin Tech, Korea. And shakers (SK-600 and ViBa 330) ware purchased from JEIO TECH (Korea) and Collomix GmbH (Gaimersheim, Germany). Pesticide product was used 42% SC (Suspension Concentrate), product name “Tel-Do” (Bayer Crop Science, Korea) (Table 2).
Field test
The ‘Hayward’ variety was cultivated in the test field located at Doyeon 2-dong, Jeju-si, Jeju Special Self-Governing Province, Korea. The test plot area was 10 m2 for each replicate and four test groups (30-21, 21-14, 14-7, 7-0 days before harvest) with triplicate plot were sprayed by pesticide. To prevent pesticides from scattering, the buffer zone was designed to be 2 m2, respectively. The temperature and humidity in the greenhouse during cultivation were continuously recorded by EL-USB-2-LCD+ (LASCAR, USA). The untreated samples were collected before pesticide application. And pesticide was sprayed using a MARUYAMA pressurized sprayer at 0, 7, 14, 21, 30 days before harvest, and pesticide was sprayed twice with 7 days interval for each treatment group of 30-21, 21-14, 14-7, 7-0 days before harvest.
Sample preparation
Kiwi fruits were harvested from 21, 14, 7, 0 days after final treatment. Samples were randomly collected about 2 kg from each replication plot. After sampling, fruits were put into kraft paper bag and transported to the laboratory, where weight was measured and fruits were cut into 8-pieces and stored in deep freezer at -70oC for 1 day. After one day, those were homogenized using a mixer and stored in a freezer at lower -20oC until analysis. Also, kiwi fruits harvested were stored in two different temperatures. One group was stored at room temperature (25oC) for 8 days, and the other group was stored at refrigeration temperature (4oC) for 21 days. Each group was conducted in 3 repetitions, and 5 samples were stored for each repetition. Samples stored at room temperature were homogenized using a blender after 0, 3, 5, and 8 days, respectively. Samples stored at refrigerated temperature were homogenized using a blender after 0, 4, 8, 12, 15 and 21 days, respectively. Homogenized samples were stored in the freezer until analysis.
Calibration curve and linearity
Fenhexamid standard (10.07 mg, 99.3%) was placed in a 10 mL volumetric flask, and messed up with acetonitrile to make stock solution of 1,000 mg/L. This solution was diluted with acetonitrile to prepare 100 mg/L standard solution. One milliliter of standard solution was transferred in a 10 mL volumetric flask and 10 mg/L standard solution was prepared. Dilution with acetonitrile was done to prepare 0.005, 0.01, 0.02, 0.05, 0.1, 0.2 mg/L solutions. Matrix matched standard was prepared by redissolving 0.005, 0.01, 0.02, 0.05, 0.1, 0.2 mg/L standard solutions dissolved in acetonitrile with nitrogen-dried and untreated kiwi samples. The standard product prepared as above 2 μL of was injected into LC-MS/MS to display on the chromatogram. The calibration line was created using the bracket calibration method and based on the peak area value.
MLOQ and sample preparation
The method limit of quantitation (MLOQ) in an analytical method refers to a numerical value calculated by considering the limit of quantitation, sample amount, and dilution factor, and represents the limit of reliable quantitation as an overall analytical method used in the study. The MLOQ in this study is the same as equation (1), and Bachmann and Nublein (1995) reported that the MLOQ in grapes was 0.02 mg/kg, and in strawberries, cherries, plums and peaches, 0.05 mg/kg (Bachmann and Nublein, 1995). Cabras et al. (2001) reported a limit of quantitation of 0.1 mg/kg and Hengel et al. (2003) reported a limit of quantitation of 0.02 mg/kg, and the MLOQ in this study was 0.01 mg/kg, which was equal to or lower than the reported MLOQ. Kwi sample was fortified by standard solution of fenhexamid to make each residue level of MLOQ (0.01 mg/kg), 10 MLOQ (0.1 mg/kg).
| (1) |
20 g of the homogenized sample was taken, and 100 mL of acetone was added, followed by extraction for 30 minutes on a shaker (200 rpm). The extract was suction filtered on a Büchner funnel, washed with 50 mL acetone the residue and the container were combined with the previous filtrate. The filtration was transferred to a 1,000 mL separatory funnel, 50 mL saturated brine and 450 mL distilled water were sequentially added, followed by extraction twice with 70 mL of dichloromethane. The dichloromethane extract was passed through 20 g of anhydrous sodium sulfate, dehydrated, concentrated under reduced pressure in a 40oC water bath, dried, redissolved in 5 mL of hexane (0.1% acetic acid), and used in the purification process. 1 mL of the sample re-dissolved in 5 mL of hexane (0.1% acetic acid) was added dropwise to SPE-florisil (1 g) activated with 5 mL of hexane (0.1% acetic acid) was eluted to 10 mL. Immediately after concentration, the residue was redissolved in 4 mL of acetone and injected into LC-MS/MS at 2 μL to calculate the residual amount by comparing the peak area on the chromatogram to the standard calibration curve.
Instrumental condition
High-performance liquid chromatography system (Shimadzu LC-MS 8040 series) equipped with a quadruple spectrometer was used for fenhexamid analysis. The kiwi samples extracts were separated on a Shiseido CAPCELL CORE C18 column (150 mm × 2.1 mm, 2.7 μm-particle) at 40oC. The mobile phase of pump A was distilled water containing 0.1% formic acid, 5 mM ammonium formate, whereas that for pump B was methanol containing 0.1 % formic acid, 5 mM ammonium formate. The mobile phase using an isocratic system consisted of A (25%), B (75%). The flow rate was 0.2 mL/min. The 2 μL was injected via the autosampler. In the case of MSMS, nebulizing gas flow was 3 mL/min, CID gas was 230 kPa, interface voltage was 4.5 kV (Table 3), and MRM conditions were as follows (Table 4).
Reduction rate
Kiwi is a fruit that is consumed after a post-ripening process. To measure the rate of reduction rate during storage according to storage temperature, the Eq. (2) was used.
| (2) |
Results and Discussions
Method validation
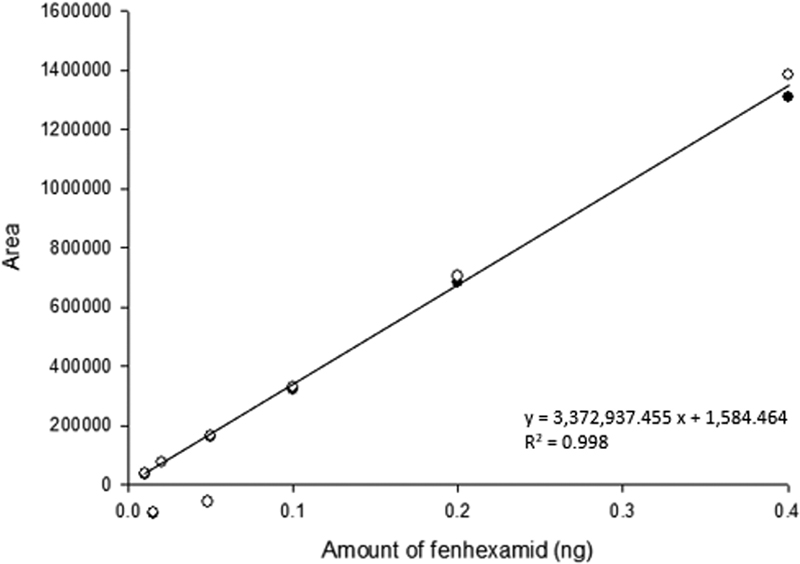
The standard calibration curve equation from 0.01 ng to 0.4 ng of fenhexamid was y = 3,372,937.455x + 1,584.464, and the correlation coefficient was 0.998. The correlation coefficient was very close to 1 and is in an acceptable range (r2 > 0.99) (Fig. 1).
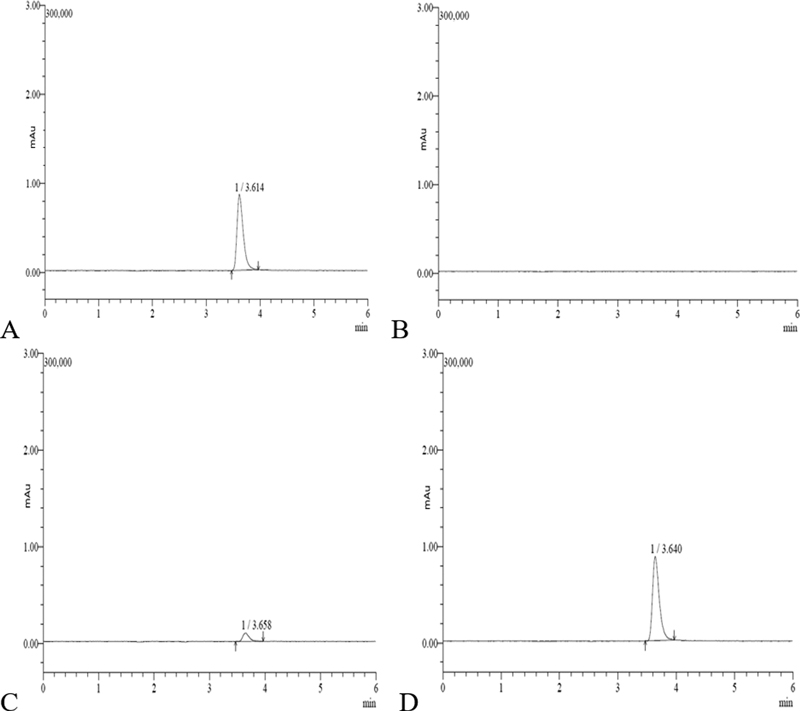
The recovery rate of the residual analysis method for kiwi of fenhexamid showed 100.3 to 105.9% at the level of 0.01 mg/kg and 103.7 to 104.6% at the level of 0.1 mg/kg, and the quantitative limit was 0.01 mg/kg. The coefficient of variation is 2.8% at the level of 0.01 mg/kg and 0.5% at the level of 0.1 mg/kg, which is included in the allowable range of accuracy and repeatability (RDA, 2019). A summary of recovery results is shown in Table 5, and it was confirmed that there was no interfering substance in the control sample of the kiwi (Fig. 2). Hengel et al. (2003) reported recovery rates ranging from 74 to 96% as a result analyzing cranberry, blueberry, and pomegranate after adding the standard solution to untreated samples using LCMS/MS. Also, Lee et al. (2009) showed 88.3 to 94.8% recovery rate test results in Korean cabbage, apple and tangerine, and red pepper.
Residual characteristics and patterns of fenhexamid in kiwi
The maximum residual amount of fenhexamid was 1.03 mg/kg in the 30-21 treated group, 1.43 mg/kg in the 21-14 treated group, 3.25 mg/kg in the 14-7 treated group, and 4.69 mg/kg in the 7-0 treated group (Table 6). Fenhexamid’s residual pattern tended to decrease over time. All treated groups were lower than the MRL level (15†mg/kg). As a result of analyzing the samples harvested 0, 7, 14, and 21 days after the final treatment, the average was 4.06, 2.51, 1.34, 0.96 mg/kg for each test group, and compared with the initial residual amount, it was confirmed that it decreased by 38.1% after 7 day, by 70.0% after 14 days and by 76.4% after 21 days. In this study, results like the report that the residual amount increased in the case of samples sprayed with pesticides near the harvest time were found (Han et al., 2004). Jin et al. (2018) found that the fenhexamid in chives was 14.58 mg/kg on 0 day, 8.95 mg/kg after 3 days, 7.81 mg/kg after 7 days, and 5.28 mg/kg after 14 days, and the fenhexamid in spinach was 15.20 mg/kg, 12.55 mg/kg, 8.89 mg/kg, 5.93 mg/kg. Also, the fenhexamid was 17.49 mg/kg, 14.69 mg/kg, 7.36 mg/kg, and 4.94 mg/kg in Korean cabbage, and 7.32 mg/kg, 6.07 mg/kg, 2.99 mg/kg, and 1.34 mg/kg in shallot. It has been reported that the residual amount of pesticides in crops is affected by several factors such as cultivation conditions, loss by rain, photodegradation, physicochemical properties of pesticides, and dilution effect by hypertrophic growth (Lee et al., 2003). The initial residual amount is thought to be relatively large in the amount of pesticides attached to leafy vegetables because the surface area of the leaves is wide and spread out. Among leafy vegetables, the fenhexamid showed a tendency to decrease by about 50% 7 days after the final drug treatment. It is judged that the dilution effect by growth was applied compared to kiwi.
Residual characteristic of fenhexamid in kiwi according to storage temperature
In the case of kiwi stored at room temperature (25oC), the initial residual concentration was 4.32 mg/kg on average. After three days, the tendency to gradually decreased to 4.03 mg/kg, six days to 3.72 mg/kg, and eight days to 3.64 mg/kg was observed. Residual concentration decreased by 6.9% after three days, 14.1% after six days, and 15.7% after eight days, compared to the initial concentration (Table 7). On the other hand, for kiwi stored at refrigerated temperature (4oC), the initial residual concentration was 4.37 mg/kg, 4.01 mg/kg after four days, 4.03 mg/kg after eight days, 3.91 mg/kg after 12 days, 4.04 mg/kg after 15 days, and 2.64 mg/kg after 21 days. Finally, it was confirmed that the tendency to decrease compared to the initial concentration. Compared to the initial concentration, it was found to be significantly reduced to 8.4% after 4 days, 8% after 8 days, 10.7% after 12 days, 7.7% after 15 days, and 39.7% after 21 days (Table 7).

Change in residual patterns of fenhexamid in kiwi according to post-harvest storage at different temperatures
To check the residual amount of fenhexamid during the ripening process, it was aged under two conditions: room temperature (25oC) and refrigeration (4oC). When aged for 8 days at room temperature, it decreased by about 15.7% compared to the initial residual amount, and when it was aged for 8 days in refrigeration, it decreased by about 8%. When stored in refrigeration for the same period, the reduction rate of pesticides was lower than when stored at room temperature. The decomposition factors of pesticides are known as changes in storage temperature, time, heating, and acidity. Cha et al. (1995) stated that agricultural products undergo decomposition due to respiration and enzyme action during storage after harvest and are more active at room temperature than at low temperatures because they are affected by temperature. Lee et al. (2003) found that the decomposition rate of drugs was slower when stored at low temperature than when stored at room temperature, so that the residual amount of pesticides in crops was affected depending on the storage conditions and period.
Conclusions
During kiwi cultivation, when fenhexamid is used twice at an interval of 7 days before harvest, it remains at a level that does not exceed the MRL standard, so it is expected that safer agricultural production will be possible. When stored at room temperature, it is effective in terms of reducing pesticides, but there is a possibility that the value of the product may decrease in terms of moisture reduction and poor quality in appearance. Thus, as a result of comparing the residual amount according to room temperature and refrigerated storage (8 days), in the case of post-ripened fruits, it is considered appropriate to refrigerate if post-ripening has occurred, and store at room temperature if post-ripening has not occurred.
Acknowledgments
This research was funded by a grant (No. PJ014491) from Rural Development Administration, Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
-
Abd-Alrahman SH, 2014. Residue and dissipation kinetics of thiamethoxam in a vegetable-field ecosystem using QuEChERS methodology combined with HPLC-DAD. Food Chem. 159:1-4.
[https://doi.org/10.1016/j.foodchem.2014.02.124]

-
Angioni A, Porcu L, Dedola F, 2011. Determination of famoxadone, fenamidone, fenhexamid and iprodione residues in greenhouse tomatoes. Pest. Manag. Sci. 68(4):543-547.
[https://doi.org/10.1002/ps.2287]

-
Arias-Estévez M, López-Periago E, Martínez-Carball E, Simal-Gándara J, Mejuto JC, et al., 2018. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric., Ecosyst. Environ. 123(4): 247-260.
[https://doi.org/10.1016/j.agee.2007.07.011]

- Bachmann J, Nublein F, 1995. Method for the determination of KBR 2738 residues in plant material by HPLC, MR-144/94, Bayer AG, Germany.
- Cha KS, Lim CW, Kim SJ, Jung IC, Moon YH, 1995. A study on elimination of captan residues stuck on spinach. J. Korean Soc. Food Nutr. 24(2):214-218. (In Korean)
-
Esteve-Turrillas FA, Abad-Fuentes A, Mercader JV, 2011. Determination of fenhexamid residues in grape must, kiwifruit, and strawberry samples by enzyme-linked immunosorbent assay. Food Chem. 124(4):1727-1733.
[https://doi.org/10.1016/j.foodchem.2010.07.112]

- Han SS, Lo SC, Kim WJ, Park PJ, Kim IK, 2003. Gas Chromatographic analysis on the residual of fungicide fenhexamid in crops (Cucumber, Strawberry and Grape). Anal. Sci. Technol. 16(1):70-77.
-
Han SS, Ma SY, Lo SC, 2004. Effect of some variation factors on dissipation of tebuconazole in grape. Korean J. Environ. Agric. 23(3):142-147.
[https://doi.org/10.5338/KJEA.2004.23.3.142]

-
Hengel M, Hung B, Engerbretson J, Shibamoto T, 2003. Analysis of fenhexamid in caneberry, blueberry, and pomegranate by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 51(23):6635-6639.
[https://doi.org/10.1021/jf0301403]

-
Jin MJ, Park HK, Jeong HR, Lee JW, Jo SH, et al., 2018. Residual characteristics and safety assessments of the fungicide fenhexamid in some minor crops. Korean J. Pestic. Sci. 22(4):363-369.
[https://doi.org/10.7585/kjps.2018.22.4.363]

- KCPA, 2015. Guideline of Pesticides Use, Korea. 321-324.
-
Lassalle Y, Kinani A, Rifai A, Souissi Y, Clavaguera C, et al., 2014. UV‐visible degradation of boscalid-structural characterization of photoproducts and potential toxicity using in silico tests. Rapid Commun. Mass Spectrom. 28(10):1153-1163.
[https://doi.org/10.1002/rcm.6880]

- Lee HR, Riu MJ, Park HW, Na YR, Song HH, et al., 2009. Establishment of analytical method for fenhexamid residue in Korean cabbage, apple, mandarin and green pepper. Korean J. Pestic. Sci. 13(4):223-231.
-
Lee YJ, Ko KY, Won DJ, Gil GH, Lee KS, 2003. Residue patterns of procymidone, chlorpyrifos and cypermethrin in peaches during cultivation and storage period. Korean J. Environ. Agric. 22(3):8~.
[https://doi.org/10.5338/KJEA.2003.22.3.220]

- NIHHS. Status of Domestic Kiwi Industry. National Institute of Horticultural & Herbal Science. http://www.nihhs.go.kr/usr/main/mainPage.do, .2017.
-
Ormad MP, Miguel N, Claver A, Matesanz JM, Ovelleiro JL, 2008. Pesticides removal in the process of drinking water production. Chemosphere 71(1):97-106.
[https://doi.org/10.1016/j.chemosphere.2007.10.006]

- The Pesticide Manual, 18th ed.; Turner, J.A.; British Crop Production Council: Hampshire, England, 2018; pp. 455-456.
Jeong-Hun Sun, Hansalim Agro-Food Analysis Center, Hankyong National University Industry Academic Cooperation Foundation, Researcher, https://orcid.org/0000-0003-1531-9690
Hyeong-Wook Jo, Hansalim Agro-Food Analysis Center, Hankyong National University Industry Academic Cooperation Foundation, Researcher, https://orcid.org/0000-0002-2271-9767
Joon-Kwan Moon, Department of Plant Resources and Landscape Architecture, Hankyong National University, Professor, https://orcid.org/0000-0001-9944-7475
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, J.H.S. and J.K.M.; methodology, J.H.S.; software, J.H.S; validation, J.H.S.; investigation, J.H.S.; resources, J.H.S. and J.K.M.; data curation, H.W.J. and J.K.M.; writing—original draft preparation, J.H.S.; writing—review and editing, H.W.J. All authors have read and agreed to the published version of the manuscript.”.
This paper was written based on the first author’s master’s thesis.